Outer membrane protein OpcA
Outer membrane adhesin OpcA protein family consists of several Neisseria species specific outer membrane proteins. Neisseria meningitidis causes meningococcal meningitis and sepsis. Opc (formerly called 5C) is one of the major outer membrane proteins and has been shown to play an important role in meningococcal adhesion and invasion of epithelial and endothelial cells, mediating attachment to host cells by binding proteoglycan cell-surface receptors.[1]
| Outer membrane protein OpcA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | OpcA | ||||||||
| Pfam | PF07239 | ||||||||
| InterPro | IPR009876 | ||||||||
| SCOPe | 1k24 / SUPFAM | ||||||||
| OPM superfamily | 240 | ||||||||
| OPM protein | 1k24 | ||||||||
| |||||||||
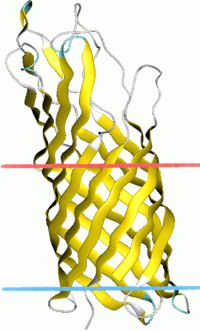
OpcA forms a 10-stranded beta-barrel with five highly mobile extracellular loops that protrude above the surface of the membrane.[2] These extracellular loops combine to form a crevice in the external surface that is lined by positively charged residues, which is predicted to be a binding site for proteoglycan polysaccharides involved in pathogenesis. Conformational changes in the extracellular loops modulate the surface of OpcA, which could affect the proteoglycan binding site.[3] These conformational changes could also lead to pore opening.
References
- Zhu P, Klutch MJ, Derrick JP, Prince SM, Tsang RS, Tsai CM (2003). "Identification of opcA gene in Neisseria polysaccharea: interspecies diversity of Opc protein family". Gene. 307: 31–40. doi:10.1016/S0378-1119(02)01208-8. PMID 12706886.
- Derrick JP, Prince SM, Achtman M (2002). "Crystal structure of the OpcA integral membrane adhesin from Neisseria meningitidis". Proc. Natl. Acad. Sci. U.S.A. 99 (6): 3417–21. doi:10.1073/pnas.062630899. PMC 122538. PMID 11891340.
- Sansom MS, Derrick JP, Bond PJ (2007). "Membrane Simulations of OpcA: Gating in the Loops?". Biophys. J. 92 (2): L23–5. doi:10.1529/biophysj.106.097311. PMC 1751375. PMID 17114231.