Organosilanols
Organosilanols are a group of chemical silicon compounds. More specifically, they are carbosilanes derivatized with a hydroxy group on the silicon atom.[1] Organosilanols are the silicon analogs to alcohols. Silanols are more acidic and more basic than their alcohol counterparts and therefore show a rich structural chemistry characterized by hydrogen bonding networks which are particularly well studied for silanetriols.[2],[3]
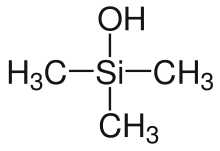
Preparation
Organosilanols can be obtained by hydrolysis of organohalosilanes, such as chlorotrimethylsilane. They can also be prepared by the oxidation of organosilanes with oxidizing agents or by hydrolysis in the alkaline.[4]
The hydrolysis of silyl ethers generally proceeds only slowly:[4]
Hydrolysis of organosilanes is a first-order reaction. The hydrolysis rate of the Si-H bond depends on the type and number of organic residues. Thus, the hydrolysis rate of trialkylsilanes is significantly slower than that of triarylsilanes. This can be explained by a stronger increase in electron density on the silicon atom by the alkyl groups. Correspondingly, the reaction rate of the tri-n-alkylsilanes decreases in the series of ethyl, propyl, butyl groups. Trialkylsilanes with n-alkyl residues react by a factor of 10 faster than the analogous silanes with branched alkyl residues.[5]
Classification
Depending on the substitution pattern of the silicon atom, a further distinction can be made. Organosilanols are classified as:
- organosilanetriols, when three hydroxy groups and an organic residue are bound to a silicon atom, e. g. methylsilanetriol, phenylsilanetriol
- organosilandiols, when two hydroxy groups and two organic residues are bound to a silicon atom, e. g. dimethylsilanediol, diphenylsilanediol
- organosilanols, when one hydroxy group and three organic residues are bound to a silicon atom, e. g. trimethylsilanol, triethylsilanol or triphenylsilanol.
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "silanols". doi:10.1351/goldbook.S05664
- Paul D. Lickiss (1995), "The synthesis and structure of organosilanols", Advances in Inorganic Chemistry, 42, pp. 147–262, doi:10.1016/S0898-8838(08)60053-7
- Rudolf Pietschnig, Stefan Spirk (2016), "The Chemistry of Organo Silanetriols", Coordination Chemistry Reviews, 323, pp. 87–106, doi:10.1016/j.ccr.2016.03.010
- Barry Arkles. "Silanes" (pdf). Gelest. p. 50. Retrieved 2016-12-10.
- G. Schott, C. Harzdorf (October 1960), "Silane. I Alkalische Solvolyse von Triorganosilanen", Zeitschrift für anorganische und allgemeine Chemie (in German), 306 (3–4), pp. 180–190, doi:10.1002/zaac.19603060306
| Wikimedia Commons has media related to Organosilanole. |