Obatoclax
Obatoclax mesylate, also known as GX15-070, is an experimental drug for the treatment of various types of cancer. It was discovered by Gemin X, which was acquired by Cephalon, which has since been acquired by Teva Pharmaceuticals.[1] Several Phase II clinical trials were completed that investigated use of Obatoclax in the treatment of leukemia, lymphoma, myelofibrosis, and mastocytosis.[2][3][4]
 | |
| Names | |
|---|---|
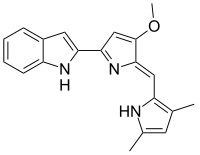
| IUPAC name
2-(2-((3,5-Dimethyl-1H-pyrrol-2-yl)methylene)-3-methoxy-2H-pyrrol-5-yl)-1H-indole | |
| Other names
GX15-070 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C20H19N3O | |
| Molar mass | 317.392 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism of action
Obatoclax is an inhibitor of the Bcl-2 family of proteins.[5] This inhibition induces apoptosis in cancer cells, preventing tumor growth. Solubility has been an issue in the development of the drug.[6]
Clinical trials
Clinical trial results have been published for treatment of acute myeloid leukemia,[7] small cell lung cancer,[8] Hodgkin's lymphoma,[9] myelodysplastic syndromes,[10]
Teva halted a phase III trial in patients with lung cancer before it had begun, citing "business decisions" as the reason.[11]
See also
References
- Cephalon Announces Definitive Agreement to Acquire Gemin X, March 21, 2011
- Parikh, Sameer A.; Kantarjian, Hagop; Schimmer, Aaron; Walsh, William; Asatiani, Ekatherine; El-Shami, Khaled; Winton, Elliott; Verstovsek, Srdan (2010). "Phase II Study of Obatoclax Mesylate (GX15-070), a Small-Molecule BCL-2 Family Antagonist, for Patients with Myelofibrosis". Clinical Lymphoma, Myeloma & Leukemia. 10 (4): 285–9. doi:10.3816/CLML.2010.n.059. PMID 20709666.
- Gemin X Presents New Data on Obatoclax at the American Society of Hematology Meeting, Dec 9, 2008
- Obatoclax at ClinicalTrials.gov
- Konopleva, M.; Watt, J.; Contractor, R.; Tsao, T.; Harris, D.; Estrov, Z.; Bornmann, W.; Kantarjian, H.; Viallet, J.; Samudio, I.; Andreeff, M. (2008). "Mechanisms of Antileukemic Activity of the Novel Bcl-2 Homology Domain-3 Mimetic GX15-070 (Obatoclax)". Cancer Research. 68 (9): 3413–20. doi:10.1158/0008-5472.CAN-07-1919. PMC 4096127. PMID 18451169.
- Nguyen, Mai; Cencic, Regina; Ertel, Franziska; Bernier, Cynthia; Pelletier, Jerry; Roulston, Anne; Silvius, John R.; Shore, Gordon C. (2015). "Obatoclax is a direct and potent antagonist of membrane-restricted Mcl-1 and is synthetic lethal with treatment that induces Bim". BMC Cancer. 15. doi:10.1186/s12885-015-1582-5. PMC 4522062.
- Schimmer, Aaron D.; Raza, Azra; Carter, Thomas H.; Claxton, David; Erba, Harry; Deangelo, Daniel J.; Tallman, Martin S.; Goard, Carolyn; Borthakur, Gautam (2014). "A Multicenter Phase I/II Study of Obatoclax Mesylate Administered as a 3- or 24-Hour Infusion in Older Patients with Previously Untreated Acute Myeloid Leukemia". PLoS ONE. 9 (10): e108694. Bibcode:2014PLoSO...9j8694S. doi:10.1371/journal.pone.0108694. PMC 4186779. PMID 25285531.
- Langer, Corey J.; Albert, Istvan; Ross, Helen J.; Kovacs, Peter; Blakely, L. Johnetta; Pajkos, Gabor; Somfay, Attila; Zatloukal, Petr; Kazarnowicz, Andrzej; Moezi, Mehdi M.; Schreeder, Marshall T.; Schnyder, Judy; Ao-Baslock, Ada; Pathak, Ashutosh K.; Berger, Mark S. (2014). "Randomized phase II study of carboplatin and etoposide with or without obatoclax mesylate in extensive-stage small cell lung cancer". Lung Cancer. 85 (3): 420–8. doi:10.1016/j.lungcan.2014.05.003. PMID 24997137.
- Oki, Y.; Copeland, A.; Hagemeister, F.; Fayad, L. E.; Fanale, M.; Romaguera, J.; Younes, A. (2012). "Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma". Blood. 119 (9): 2171–2. doi:10.1182/blood-2011-11-391037. PMID 22383790.
- Arellano, Martha L.; Borthakur, Gautam; Berger, Mark; Luer, Jill; Raza, Azra (2014). "A Phase II, Multicenter, Open-Label Study of Obatoclax Mesylate in Patients with Previously Untreated Myelodysplastic Syndromes with Anemia or Thrombocytopenia". Clinical Lymphoma, Myeloma & Leukemia. 14 (6): 534–9. doi:10.1016/j.clml.2014.04.007. PMID 25052051.
- Clinical trial number NCT01563601 for "Efficacy and Safety of Obatoclax Mesylate in Combination With Carboplatin and Etoposide Compared With Carboplatin and Etoposide Alone in Chemotherapy-Naive Patients With Extensive-Stage Small Cell Lung Cancer" at ClinicalTrials.gov