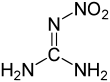
Nitroguanidine
Nitroguanidine- abbreviated NGu or NQ- is a colorless, crystalline solid that melts at 257 °C and decomposes at 254 °C. NGu is an extremely insensitive but powerful high explosive. Wetting NGu with > 20 wt.-% water effects desensitization from HD 1.1 down to HD 4.1 (flammable solid)[1]. NGu is used as an energetic material (propellants, High explosives), precursor for insecticides, and for other purposes.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-Nitroguanidine | |||
| Other names
Picrite | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.313 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH4N4O2 | |||
| Molar mass | 104.07 g/mol | ||
| Appearance | Colorless crystalline solid | ||
| Density | 1.77 g/cm3 | ||
| Melting point | 257 °C (495 °F; 530 K) | ||
| 3.45 g/kg (in water at 25 °C) | |||
| Explosive data | |||
| Shock sensitivity | > 50 J | ||
| Friction sensitivity | > 350 N | ||
| RE factor | 1.00 | ||
| Hazards | |||
| Main hazards | Explosive | ||
| Related compounds | |||
Related compounds |
Guanidine Guanidine nitrate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Manufacture
Nitroguanidine is produced worldwide on a large scale starting with the reaction of dicyandiamide (DCD) with ammonium nitrate to afford the salt guanidinium nitrate, GN. GN is nitrated by treatment with concentrated sulfuric acid at low temperature.[2]
- [C(NH2)3]NO3 → (NH2)2CNNO2 + H2O
Nitroguanidine can also be generated by treatment of urea with ammonium nitrate (BMR-process). However, owing to problems of reliability and safety, this process has never been commercialized despite its attractive economic features.
Uses
Explosives
Nitroguanidine is in use since the 1930s as an ingredient in triple-base gun propellants in which it reduces flame temperature, muzzle flash and erosion of the gun barrel but preserves chamber pressure due to high nitrogen content. Its extreme insensitivity combined with low cost has made NGu become a popular ingredient in insensitive high explosive formulations (e.g AFX-453, AFX-760, IMX-101, AL-IMX-101, IMX-103, etc.).[3]
Nitroguanidine's explosive decomposition is given by the following equation: H4N4CO2 (s) → 2 H2O (g) + 2 N2 (g) + C (s)
Pesticides
Nitroguanidine derivatives are used as insecticides, having a comparable effect to nicotine. Derivatives include clothianidin, dinotefuran, imidacloprid, and thiamethoxam.
Biochemistry
The nitrosoylated derivative nitrosoguanidine is often used to mutagenize bacterial cells for biochemical studies.
Structure
Following several decades of debate, it could be confirmed by NMR-spectroscopy, X-ray and neutron diffraction that nitroguanidine exclusively exists as the nitroimine tautomer both in solid state and solution.[4][5][6]
References
- United Nations, Transport of Nitroguanidine, wetted, (UN 1336) in flexible IBCs, ST/SC/AC.10/C.3/2006/52, Geneva, 13 April 2006. Accessed at https://www.unece.org/fileadmin/DAM/trans/doc/2006/ac10c3/ST-SG-AC10-C3-2006-52e.pdf
- E.-C. Koch, Insensitive High Explosives: III. Nitroguanidine – Synthesis – Structure – Spectroscopy – Sensitiveness, Propellants Explos. Pyrotech. 2019, 44, 267-292.
- E.-C. Koch, Insensitive High Explosives: IV. Nitroguanidine - Initiation & detonation, Def. Tech. 2019, 15, 467-487.
- Bulusu, S.; Dudley, R. L.; Autera, J. R. (1987). "Structure of nitroguanidine: nitroamine or nitroimine? New NMR evidence from nitrogen-15 labeled sample and nitrogen-15 spin coupling constants". Magnetic Resonance in Chemistry. 25 (3): 234–238. doi:10.1002/mrc.1260250311.
- Murmann, R. K.; Glaser, Rainer; Barnes, Charles L. (2005). "Structures of nitroso- and nitroguanidine x - ray crystallography and computational analysis". Journal of Chemical Crystallography. 35 (4): 317–325. doi:10.1007/s10870-005-3252-y.
- S. Choi, Refinement of 2-Nitroguanidine by Neutron Powder Diffraction, Acta Cryst. 1981, B37, 1955-1957.