Nitrifying bacteria
Nitrifying bacteria are chemolithotrophic organisms that include species of the genera Nitrosomonas, Nitrosococcus, Nitrobacter and Nitrococcus. These bacteria get their energy by the oxidation of inorganic nitrogen compounds.[1] Types include ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Many species of nitrifying bacteria have complex internal membrane systems that are the location for key enzymes in nitrification: ammonia monooxygenase (which oxidizes ammonia to hydroxylamine), hydroxylamine oxidoreductase (which oxidizes hydroxylamine to nitric oxide - which is oxidized to nitrite by a currently unidentified enzyme), and nitrite oxidoreductase (which oxidizes nitrite to nitrate).[2]
Ecology
Nitrifying bacteria are a narrow taxonomic group in the environment, and are found in highest numbers where considerable amounts of ammonia are present (areas with extensive protein decomposition, and sewage treatment plants).[3] Nitrifying bacteria thrive in lakes and rivers streams with high inputs and outputs of sewage and wastewater and freshwater because of the high ammonia content.
Oxidation of ammonia to nitrate
Nitrification in nature is a two-step oxidation process of ammonium (NH4+) or ammonia (NH3) to nitrate (NO3−) catalyzed by two ubiquitous bacterial groups. The first reaction is oxidation of ammonium to nitrite by ammonia oxidizing bacteria (AOB) represented by the "Nitrosomonas" genus. The second reaction is oxidation of nitrite (NO2−) to nitrate by nitrite-oxidizing bacteria (NOB), represented by the "Nitrobacter" genus.[4][5]
First step nitrification - molecular mechanism
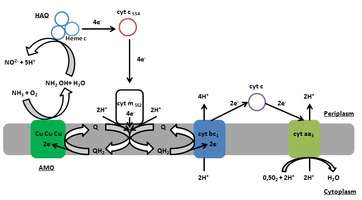
Ammonia oxidation in autotrophic nitrification is a complex process that requires several enzymes, proteins and presence of oxygen. The key enzymes, necessary to obtaining energy during oxidation of ammonia to nitrite are ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase (HAO). First is a transmembrane copper protein which catalyzes the oxidation of ammonia to hydroxylamine (1.1) taking two electrons directly from the quinone pool. This reaction requires O2.
The second step of this process has recently fallen into question.[6]
For the past few decades, the common view was that a trimeric multiheme c-type HAO converts hydroxylamine into nitrite in the periplasm with production of four electrons (1.2). The stream of four electron are channelled through cytochrome c554 to a membrane-bound cytochrome c552. Two of the electrons are routed back to AMO, where they are used for the oxidation of ammonia (quinol pool). The remaining two electrons are used to generate a proton motive force and reduce NAD(P) through reverse electron transport.[7]
Recent results, however, show that HAO does not produce nitrite as a direct product of catalysis. This enzyme instead produces nitric oxide and three electrons. Nitric oxide can then be oxidized by other enzymes (or oxygen) to nitrite. In this paradigm, the electron balance for overall metabolism needs to be reconsidered.[6]
Second step nitrification - molecular mechanism
Nitrite produced in the first step of autotrophic nitrification is oxidized to nitrate by nitrite oxidoreductase (NXR)(2). It is a membrane-associated iron-sulfur molybdoprotein, and is part of an electron transfer chain which channels electrons from nitrite to molecular oxygen. The molecular mechanism of oxidation nitrite is less described than oxidation ammonium. In new research (e.g. Woźnica A. et al., 2013)[8] proposed new hypothetical model of NOB electron transport chain and NXR mechanism (Figure 2.). In contrast to earlier models [9] the NXR acts on the outside of the plasma membrane, directly contributing to postulated by Spieck [10] and coworkers mechanism of proton gradient generation. Nevertheless, the molecular mechanism of nitrite oxidation is an open question.
Characteristic of ammonia and nitrite oxidizing bacteria
Nitrifying bacteria that oxidize ammonia [4][11]
| Genus | Phylogenetic group | DNA (mol% GC) | Habitats | Characteristics |
|---|---|---|---|---|
| Nitrosomonas | Beta | 45-53 | Soil, Sewage, freshwater, Marine | Gram-negative short to long rods, motile (polar flagella)or nonmotile; peripheral membrane systems |
| Nitrosococcus | Gamma | 49-50 | Freshwater, Marine | Large cocci, motile, vesicular or peripheral membranes |
| Nitrosospira | Beta | 54 | Soil | Spirals, motile (peritrichous flagella); no obvious membrane system |
Nitrifying bacteria that oxidize nitrite [4][11]
| Genus | Phylogenetic group | DNA (mol% GC) | Habitats | Characteristics |
|---|---|---|---|---|
| Nitrobacter | Alpha | 59-62 | Soil, Freshwater, Marine | Short rods, reproduce by budding, occasionally motile (single subterminal flagella) or non-motile; membrane system arranged as a polar cap |
| Nitrospina | Delta | 58 | Marine | Long, slender rods, nonmotile, no obvious membrane system |
| Nitrococcus | Gamma | 61 | Marine | Large Cocci, motile (one or two subterminal flagellum) membrane system randomly arranged in tubes |
| Nitrospira | Nitrospirae | 50 | Marine, Soil | Helical to vibroid-shaped cells; nonmotile; no internal membranes |
See also
References
- Mancinelli RL (1996). "The nature of nitrogen: an overview". Life Support & Biosphere Science: International Journal of Earth Space. 3 (1–2): 17–24. PMID 11539154.
- Kuypers, MMM; Marchant, HK; Kartal, B (2011). "The Microbial Nitrogen-Cycling Network". Nature Reviews Microbiology. 1 (1): 1–14. doi:10.1038/nrmicro.2018.9. PMID 29398704.
- Belser LW (1979). "Population ecology of nitrifying bacteria". Annu. Rev. Microbiol. 33: 309–333. doi:10.1146/annurev.mi.33.100179.001521. PMID 386925.
- Schaechter M. „Encyclopedia of Microbiology", AP, Amsterdam 2009
- Ward BB (1996). "Nitrification and ammonification in aquatic systems". Life Support & Biosphere Science: International Journal of Earth Space. 3 (1–2): 25–9. PMID 11539155.
- Caranto, Jonathan D.; Lancaster, Kyle M. (2017-07-17). "Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase". Proceedings of the National Academy of Sciences. 114 (31): 8217–8222. doi:10.1073/pnas.1704504114. ISSN 0027-8424. PMC 5547625. PMID 28716929.
- Byung Hong Kim, Geoffrey Michael Gadd (2008). Bacterial Physiology and Metabolism. Cambridge University Press.
- Woznica A, et al. (2013). "Stimulatory Effect of Xenobiotics on Oxidative Electron Transport of Chemolithotrophic Nitrifying Bacteria Used as Biosensing Element". PLOS ONE. 8 (1): e53484. Bibcode:2013PLoSO...853484W. doi:10.1371/journal.pone.0053484. PMC 3541135. PMID 23326438.
- Ferguson SJ, Nicholls DG (2002). Bioenergetic III. Academic Press.
- Spieck E, et al. (1998). "Isolation and immunocytochemical location of the nitrite-oxidizing system in Nitrospira moscoviensis". Arch Microbiol. 169 (3): 225–230. doi:10.1007/s002030050565. PMID 9477257.
- Michael H. Gerardi (2002). Nitrification and Denitrification in the Activated Sludge Process. John Wiley & Sons.