Nitarsone
Nitarsone is an organoarsenic compound that is used in poultry production as a feed additive to increase weight gain, improve feed efficiency, and prevent histomoniasis (blackhead disease).[1] It is marketed as Histostat by Zoetis.[2]
 | |
| Names | |
|---|---|
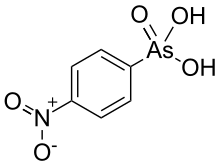
| IUPAC name
(4-Nitrophenyl)arsonic acid | |
| Other names
(p-Nitrophenyl)arsonic acid; 4-Nitrobenzenearsonic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.451 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6AsNO5 | |
| Molar mass | 247.038 g·mol−1 |
| Melting point | 298–300 °C (568–572 °F; 571–573 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitarsone once was one of four arsenical food-animal drugs—along with roxarsone, arsanilic acid, and carbarsone—approved by the U.S. Food and Drug Administration for use in feeding poultry.[3] However, following the suspension of sales of roxarsone in the United States in 2011, nitarsone was thought to be the only arsenical animal drug actually marketed in the U.S.[3][4] In September 2013, the FDA announced that Zoetis and Fleming Laboratories agreed to voluntarily withdraw from using roxarsone, arsanilic acid, and carbarsone, which left nitarsone as the only arsenical approved in the U.S. for use in food animals.[5] But in 2015, the FDA also withdrew approval of nitarsone in animal feeds, effective at the end of 2015.[6]
References
- U.S. Food and Drug Administration. "Animal Drugs @ FDA".
- Zoetis. "Histostat: Type A Medicated Feed Article".
- U.S. Food and Drug Administration (June 8, 2011). SafetyHealth/ProductSafetyInformation/ucm258313.htm "Questions and Answers Regarding 3-Nitro (Roxarsone)" Check
|url=value (help). - Sabrina Tavernise (May 11, 2013). health/study-finds-an-increase-in-arsenic-levels-in-chicken.html "Study Finds an Increase in Arsenic Levels in Chicken" Check
|url=value (help). New York Times. - U.S. FDA (September 20, 2011). ProductSafetyInformation/ucm370568.htm "FDA Response to Citizen Petition on Arsenic-based Animal Drugs" Check
|url=value (help). - U.S. Food and Drug Administration (April 1, 2015). "FDA Announces Pending Withdrawal of Approval of Nitarsone".