Nickel hydrazine nitrate
Nickel hydrazine nitrate (NHN), (chemical formula: [Ni(N2H4)3](NO3)2 is an energetic material having explosive properties in between that of primary explosive and a secondary explosive.[1] It is a salt of a coordination compound of nickel with a reaction equation of 3N2H4·H2O + Ni(NO3)2 →〔Ni(N2H4)3〕(NO3)2 + 3H2O[2]
 Properly made (talcum powder like consistency) [3]
Properly made (talcum powder like consistency) [3] Improperly made (Hard chunks, grains of sand consistency)
Improperly made (Hard chunks, grains of sand consistency)
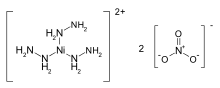 | |
| Names | |
|---|---|
| Other names
Tris(hydrazine)nickel(II) nitrate Trihydrazinenickel nitrate NHN | |
| Identifiers | |
| Properties | |
| H12N8NiO6 | |
| Molar mass | 278.839 g·mol−1 |
| Appearance | purple solid |
| Boiling point | explode |
| Explosive data | |
| Shock sensitivity | 18.82 J |
| Friction sensitivity | 15.6906 N |
| Detonation velocity | 3,600 m/s @ .8 g/cm3
7,000 m/s @ 1.7 g/cm³ |
| RE factor | 1.05 @ 1.7 g/cm3 |
| Hazards | |
EU classification (DSD) (outdated) |
Oxidant (O) Carc. Cat. 1 Muta. Cat. 3 Repr. Cat. 2 Toxic (T) Harmful (Xn) Irritant (Xi) Dangerous for the environment (N) |
| NFPA 704 (fire diamond) | |
| Explosive limits | 219°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
NHN can be synthesized by reacting nickel(II) nitrate hexahydrate with a dilute aqueous solution of hydrazine monohydrate at 65 C.[4] To help speed the drying of the product after filtration from the hot water, it can be rinsed with alcohol. The product is a fluffy powder (density=0.9 g/cm3). To increase its bulk density to (1.2 g/cm3), dextrin in the amount of (1%) of the weight of the nickel(II) nitrate hexahydrate can be added.[5]
Non-primary explosive detonator (NPED)
The sensitivity of NHN straddles the line between highly sensitive primaries and a sensitive secondary. Due to this fact, NHN can be considered a true Non-primary explosive detonator. (NPED)
An added benefit of NHN is that it will make the DDT (Deflagration to Detonation) transition in a cardboard shell, thus eliminating any chance of shrapnel danger posed by using metal shells.
Safety
NHN straddles the line between primary and secondary. Because of this it is a relatively safe explosive to work with having 80x less sensitivity to friction (16.0 N) than Lead Azide (0.1N) as shown in table 2.
Friction sensitivities of some traditional explosives (lead azide – 0.1N; lead trinitroresorcinate – 1.5 N; mercury fulminate (white) – 5,0 N; tetrazene – 8.0 N; PETN – 60 N; hexogen – 120 N; octogen – 120 N, show that NHN is not very sensitive, and is thereby not exceedingly hazardous in handling.[6]
Table 1. General and structural properties of Nickel hydrazine nitrate[1]
| Molecular formula | Ni H12 N8 O6 |
| Formula weight | 278.69 |
| Color | Purple Violet |
| Crystal density (g/cm3) | 2.1 |
| Average particle size (μm) | 13 |
| Nickel content (%) | 21.16 (21.06) a |
| Hydrazine content (%) | 34.46 (34.45) a |
| Nitrate content (%) | 44.47 (44.49) a |
| Nitrogen content in coordination sphere (%) | 30.25 (30.14) a |
| FTIR peaks, (cm−1) | 3238, 1630 (NH2); 1356,1321 (-NO3) |
| Moisture content (at 333 K for 10 min) (%) | 0.34 |
| Average mol wt of combustion products | 27.35 |
| Percent condensable Ni (l) | 18 |
| Oxygen-fuel ratio | 0.8571 |
| Oxygen balance % | -5.74 |
a Values in brackets are theoretical
Table 2. Comparative properties of Nickel hydrazine nitrate and lead azide[1]
| Property | Nickel hydrazine nitrate a | Lead azide b | Lead styphnate |
|---|---|---|---|
| Crystal Density (g/cm3) | 2.129 | 4.38 | 3.02 |
| Oxygen balance (%) | – 5.74 c | – 5.50 | -19.00 |
| Heat of combustion (kJ/kg) | 5225 | 2635 | 5234 |
| Heat of formation (kJ/mol) | – 449 | 469 | -385 |
| Heat of explosion (kJ/kg) | 4390 | 1610 | 1912 |
| Pressure output in closed vessel (100 mg in 48 cm3) (kg/cm2) | 17.5 | 8.2 c | |
| Onset of decomposition (K) | 505.7 | 463 | 533.15 |
| Peak of decomposition (K) | 506.5 | 618 | 583.15 |
| Friction sensitivity (kg f) | 1.6 | 0.02 | .15 |
| Impact sensitivity (cm, 400 g wt, 20 mg sample, 50% explosion) | 21 b | 10.5 | 11 |
| ESD sensitivity (J) | 0.02 b | 0.004 | .0002 |
| Vol. of detonation gases (ml/g) | 884 c | 308 | 368 |
| Detonation temperature (K) | 2342 c | 5600 | |
| Detonation pressure (GPa) | 20.8 c (1.7 g/cm3) | 16.1 (3.0 g/cm3) | |
| Detonation velocity (m/s) | 7000 b (1.7 g/cm3) | 4630 (3.0 g/cm3) | 5200 (2.9 g/cm3) |
| RE Factor | 1.05 b (1.7 g/cm3) | .8 (3.0 g/cm3) |
a Experimental value, b literature value, and c theoretical value
References
- Hariharanath, B.; Chandrabhanu, K. S.; Rajendran, A. G.; Ravindran, M.; Kartha, C. B. (2006). "Detonator using Nickel Hydrazine Nitrate as Primary Explosive". Defence Science Journal. 56 (3): 383–9. doi:10.14429/dsj.56.1904.
- Xiang, Dong; Zhu, Weihua (15 February 2018). "Thermal decomposition of energetic MOFs nickel hydrazine nitrate crystals from an ab initio molecular dynamics simulation". Computational Materials Science. 143: 170–181. doi:10.1016/j.commatsci.2017.11.006.
- Nickel Hydrazine Nitrate (Dextrinated) https://www.youtube.com/watch?v=rPxdDSUGxo4&t=11s
- Chhabra, J.S; Talawar, M.B; Makashir, P.S; Asthana, S.N; Singh, Haridwar (2003). "Synthesis, characterization and thermal studies of (Ni/Co) metal salts of hydrazine: Potential initiatory compounds". Journal of Hazardous Materials. 99 (3): 225–39. doi:10.1016/S0304-3894(02)00247-9. PMID 12758009.
- Talawar, M B; Agrawal, A P; Chhabra, J S; Ghatak, C K; Asthana, S N; Rao, K U B (August 2004). "Studies on nickel hydrazinium nitrate (NHN) and bis-(5-nitro-2H tetrazolato-N2)tetraamino cobalt(III) perchlorate (BNCP): Potential lead-free advanced primary explosives" (PDF). Journal of Scientific & Industrial Research. 63 (8): 677–681. hdl:123456789/5478.
- Wojewódka, Andrzej; Bełzowski, Janusz (2011). "Hydrazynowe kompleksy metali przejściowych jako perspektywiczne materiały wybuchowe" [Hydrazine complexes of transition metals as perspective explosives]. Chemik. 65 (1): 20–27.
