New fuchsine
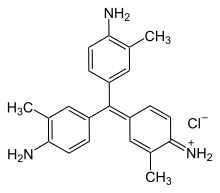
New fuchsine is an organic compound with the formula [(H2N(CH3)C6H3)3C]Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes.[1] The other is pararosaniline. It is prepared by condensation of ortho-toluidine with formaldehyde. This process initially gives the benzhydrol 4,4'-bis(dimethylamino)benzhydrol, which is further condensed to give the leuco (colorless) tertiary alcohol [(H2N(CH3)C6H3)3COH, which is oxidized in acid to give the dye.[2]
 | |
| Names | |
|---|---|
| Other names
New fuchsin; Magenta III; Basic Violet 2; C.I. 42520 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.019.847 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H24ClN3 | |
| Molar mass | 365.91 g·mol−1 |
| Appearance | violet powder |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H315, H318, H319, H335, H350, H351, H373, H400, H410 |
| P201, P202, P260, P261, P264, P271, P273, P280, P281, P302+352, P304+340, P305+351+338, P308+313, P310, P312, P314, P321, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use as dye and stain
New fuchsine is used to dye polyacrylonitrile, paper, and leather. In biology, it can be used for staining acid-fast organisms, e.g. by Ziehl-Neelsen stain, and for making Schiff's reagent. As a primary amine, the dye can be diazotized in the laboratory, and the resulting diazonium salt used as a trapping agent in enzyme histochemistry.[3]
Etymology
The name fuchsine recognizes Leonhardt Fuchs.
See also
References
- Horobin RW, Kiernan JA (2002) Conn's Biological Stains, 10th ed. Oxford: BIOS.
- Thetford, Dean; Updated By Staff (2013). "Triphenylmethane and Related Dyes". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.2018091620080520.a01.pub2. ISBN 978-0471238966.
- Lojda Z, Gossrau R, Schiebler TH (1979) Enzyme Histochemistry. A Laboratory Manual. Berlin: Springer-Verlag.