Naphtholactam
Naphtholactam is an organic compound derived from naphthalene. It is a tricyclic species consisting of a naphthalene core fused with a lactam (NH-CO-) at the 1,8-positions. The N-alkyl derivatives are commercially important.
 | |
| Names | |
|---|---|
| Other names
1,8-naphtholactam, benz[cd]indol-2(1H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.523 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H7NO | |
| Molar mass | 169.183 g·mol−1 |
| Appearance | white solid |
| Melting point | 181 °C (358 °F; 454 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302 |
| P264, P270, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dye precursor
It is a precursor to the dye anthanthrone via ring-opening to the amino carboxylic acid, which can be converted to the diazonium salt. Naphthostyril derivatiives are also of interest in medicinal chemistry.[1] Naphthostyrils can be produced by metal-catalyzed cyclization of 1-naphthylamides.[2]
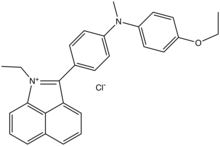
Chemical structure of typical naphtholactam dye.
N-Ethylnaphtholactam and related derivatives are precursors to many dyes. The lactam condenses with anilines in the presence of phosphorus oxychloride.[3]
gollark: The reason Minoteaur v7 needs (or, well, benefits from) GPUs is that it uses machine learning™ algorithms for the search system.
gollark: Consider, though: Minoteaur v6 was written in Nim. Nim has more libraries than Macron will for years, and yet I still felt annoyed by some stuff being missing enough to switch to Python (among other things).
gollark: Most OSes require special permissions for that.
gollark: That won't actually work.
gollark: Also nice HTML templating, SQLite, that sort of thing.
References
- López-Rodríguez, María L.; Porras, Esther; Morcillo, M. José; Benhamú, Bellinda; Soto, Luis J.; Lavandera, José L.; Ramos, José A.; Olivella, Mireia; Campillo, Mercedes; Pardo, Leonardo (2003). "Optimization of the Pharmacophore Model for 5-HT7R Antagonism. Design and Synthesis of New Naphtholactam and Naphthosultam Derivatives". Journal of Medicinal Chemistry. 46 (26): 5638–5650. doi:10.1021/jm030841r. PMID 14667218.
- Ying, Jun; Fu, Lu-Yang; Zhong, Guoqiang; Wu, Xiao-Feng (2019). "Cobalt-Catalyzed Direct Carbonylative Synthesis of Free (NH)-Benzo[cd]indol-2(1H)-ones from Naphthylamides". Organic Letters. 21 (14): 5694–5698. doi:10.1021/acs.orglett.9b02037. PMID 31246481.
- Berneth, Horst (2008). "Methine Dyes and Pigments". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_487.pub2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.