NDUFA2
NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2 is a protein that in humans is encoded by the NDUFA2 gene.[5][6] The NDUFA2 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain.[7] Mutations in the NDUFA2 gene are associated with Leigh's syndrome.[6]
Structure
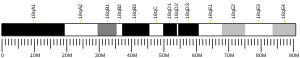
The NDUFA2 gene is located on the long (q) arm of chromosome 5 at position 31.2 and it spans 2,422 base pairs.[6] The NDUFA2 gene produces an 11 kDa protein composed of 99 amino acids.[8][9] NDUFA2 is a subunit of the enzyme NADH dehydrogenase (ubiquinone), the largest of the respiratory complexes. The structure is L-shaped with a long, hydrophobic transmembrane domain and a hydrophilic domain for the peripheral arm that includes all the known redox centers and the NADH binding site.[7] NDUFA2 is one of about 31 hydrophobic subunits that form the transmembrane region of Complex I. It has been noted that the N-terminal hydrophobic domain has the potential to be folded into an alpha helix spanning the inner mitochondrial membrane with a C-terminal hydrophilic domain interacting with globular subunits of Complex I. The highly conserved two-domain structure suggests that this feature is critical for the protein function and that the hydrophobic domain acts as an anchor for the NADH:ubiquinone oxidoreductase complex at the inner mitochondrial membrane.[6]
Function
The human NDUFA2 gene codes for a subunit of Complex I of the respiratory chain, which transfers electrons from NADH to ubiquinone. NDUFA2 is an accessory subunit of Complex I that is believed not to be involved in catalysis but may be involved in regulating Complex I activity or its assembly via assistance in redox processes.[6][10] Initially, NADH binds to Complex I and transfers two electrons to the isoalloxazine ring of the flavin mononucleotide (FMN) prosthetic arm to form FMNH2. The electrons are transferred through a series of iron-sulfur (Fe-S) clusters in the prosthetic arm and finally to coenzyme Q10 (CoQ), which is reduced to ubiquinol (CoQH2). The flow of electrons changes the redox state of the protein, resulting in a conformational change and pK shift of the ionizable side chain, which pumps four hydrogen ions out of the mitochondrial matrix.[7]
Clinical significance
Mutations in the NDUFA2 gene can result in Leigh's syndrome, a severe neurological disorder that typically arises in the first year of life.[6] One such mutation interferes with normal splicing patterns and results in exon 2 being skipped. This causes a reduction in Complex I activity and disturbs its assembly. The NDUFA2 mutation is also associated with the depolarization of the mitochondria.[11]
Interactions
NDUFA2 has many protein interactions, including interactions with other members of the NADH dehydrogenase [ubiquinone] 1 alpha subcomplex, other subunits of Complex I as well as with redox proteins. This may be due to its potential role in Complex I assembly and assistance in redox processes.[6]
References
- GRCh38: Ensembl release 89: ENSG00000131495 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000014294 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Emahazion T, Brookes AJ (Nov 1998). "Mapping of the NDUFA2, NDUFA6, NDUFA7, NDUFB8, and NDUFS8 electron transport chain genes by intron based radiation hybrid mapping". Cytogenetics and Cell Genetics. 82 (1–2): 114. doi:10.1159/000015081. PMID 9763676.
- "Entrez Gene: NDUFA2 NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 2, 8kDa".
- Pratt, Donald Voet, Judith G. Voet, Charlotte W. (2013). "18". Fundamentals of biochemistry : life at the molecular level (4th ed.). Hoboken, NJ: Wiley. pp. 581–620. ISBN 9780470547847.
- Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (Oct 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- "NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).
- "NDUFA2 - NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2". UniProt: a hub for protein information. The UniProt Consortium. Retrieved 24 March 2015.
- Hoefs SJ, Dieteren CE, Distelmaier F, Janssen RJ, Epplen A, Swarts HG, Forkink M, Rodenburg RJ, Nijtmans LG, Willems PH, Smeitink JA, van den Heuvel LP (Jun 2008). "NDUFA2 complex I mutation leads to Leigh disease". American Journal of Human Genetics. 82 (6): 1306–1315. doi:10.1016/j.ajhg.2008.05.007. PMC 2427319. PMID 18513682.
Further reading
- Walker JE, Arizmendi JM, Dupuis A, Fearnley IM, Finel M, Medd SM, Pilkington SJ, Runswick MJ, Skehel JM (Aug 1992). "Sequences of 20 subunits of NADH:ubiquinone oxidoreductase from bovine heart mitochondria. Application of a novel strategy for sequencing proteins using the polymerase chain reaction". Journal of Molecular Biology. 226 (4): 1051–72. doi:10.1016/0022-2836(92)91052-Q. PMID 1518044.
- Dunbar DR, Shibasaki Y, Dobbie L, Andersson B, Brookes AJ (1997). "In situ hybridisation mapping of genomic clones for five human respiratory chain complex I genes". Cytogenetics and Cell Genetics. 78 (1): 21–4. doi:10.1159/000134618. PMID 9345899.
- Loeffen JL, Triepels RH, van den Heuvel LP, Schuelke M, Buskens CA, Smeets RJ, Trijbels JM, Smeitink JA (Dec 1998). "cDNA of eight nuclear encoded subunits of NADH:ubiquinone oxidoreductase: human complex I cDNA characterization completed". Biochemical and Biophysical Research Communications. 253 (2): 415–22. doi:10.1006/bbrc.1998.9786. PMID 9878551.
- Zhang QH, Ye M, Wu XY, Ren SX, Zhao M, Zhao CJ, Fu G, Shen Y, Fan HY, Lu G, Zhong M, Xu XR, Han ZG, Zhang JW, Tao J, Huang QH, Zhou J, Hu GX, Gu J, Chen SJ, Chen Z (Oct 2000). "Cloning and functional analysis of cDNAs with open reading frames for 300 previously undefined genes expressed in CD34+ hematopoietic stem/progenitor cells". Genome Research. 10 (10): 1546–60. doi:10.1101/gr.140200. PMC 310934. PMID 11042152.
- Brockmann C, Diehl A, Rehbein K, Strauss H, Schmieder P, Korn B, Kühne R, Oschkinat H (Sep 2004). "The oxidized subunit B8 from human complex I adopts a thioredoxin fold". Structure. 12 (9): 1645–54. doi:10.1016/j.str.2004.06.021. PMID 15341729.
- Vogel RO, Dieteren CE, van den Heuvel LP, Willems PH, Smeitink JA, Koopman WJ, Nijtmans LG (Mar 2007). "Identification of mitochondrial complex I assembly intermediates by tracing tagged NDUFS3 demonstrates the entry point of mitochondrial subunits". The Journal of Biological Chemistry. 282 (10): 7582–90. doi:10.1074/jbc.M609410200. PMID 17209039.