Naa80
N-acetyltransferase 80 (also known as NAT6 or FUS2) is a protein that in humans is encoded by the NAA80 gene.[4] It acetylates the N-terminus of mature actin.[5]
Function
This gene encodes a member of the N-acetyltransferase family. N-acetyltransferases modify proteins by transferring acetyl groups from acetyl-CoA to the N-termini of protein substrates. The encoded protein is a cytoplasmic N-acetyltransferase with a substrate specificity for N-termini that are enriched for acidic residues.[6] This gene is located in the tumor suppressor gene region on chromosome 3p21.3, and the encoded protein may play a role in cancer. Alternatively spliced transcript variants encoding multiple isoforms have been observed. This gene overlaps and is on the same strand as hyaluronoglucosaminidase 3, and some transcripts of each gene share a portion of the first exon. [provided by RefSeq, Jan 2011].
Naa80 acetylates the N-terminus of mature actin. This N-terminal acetylation affects the rates of actin filament depolymerization and elongation, and the overall morphology of the cell.[5]
Structure
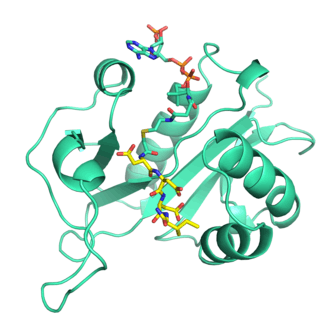
Naa80 is a member of the GNAT family of acetyltransferases.[7] It has an overall fold similar to the other N-terminal acetyltransferases, but has a more open, basic active site to accommodate the acidic N-terminus of actin. Naa80 can acetylate peptides with different N-terminal residues, but the presence of acidic residues in positions 2 and 3 is important for substrate specificity.[7][6] Most Naa80 orthologs have an extended polyproline loop which may be important for actin binding through profilin.[5][7]
References
- GRCh38: Ensembl release 89: ENSG00000243477 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: N-acetyltransferase 6 (GCN5-related)".
- Drazic, A.; Aksnes, H.; Marie, M.; Boczkowska, M.; Varland, S.; Timmerman, E.; Foyn, H.; Glomnes, N.; Rebowski, G.; Impens, F.; Gevaert, K.; Dominguez, R.; Arnesen, T. (2018). "NAA80 is actin's N-terminal acetyltransferase and regulates cytoskeleton assembly and cell motility". Proceedings of the National Academy of Sciences of the United States of America. 115 (17): 4399–4404. doi:10.1073/pnas.1718336115. PMC 5924898. PMID 29581253.
- Wiame, E.; Tahay, G.; Tyteca, D.; Vertommen, D.; Stroobant, V.; Bommer, G. T.; Van Schaftingen, E. (2018). "NAT6 acetylates the N-terminus of different forms of actin". The FEBS Journal. 285 (17): 3299–3316. doi:10.1111/febs.14605. PMID 30028079.
- Goris, M.; Magin, R. S.; Foyn, H.; Myklebust, L. M.; Varland, S.; Ree, R.; Drazic, A.; Bhambra, P.; Støve, S. I.; Baumann, M.; Haug, B. E.; Marmorstein, R.; Arnesen, T. (2018). "Structural determinants and cellular environment define processed actin as the sole substrate of the N-terminal acetyltransferase NAA80". Proceedings of the National Academy of Sciences of the United States of America. 115 (17): 4405–4410. doi:10.1073/pnas.1719251115. PMC 5924903. PMID 29581307.
Further reading
- Shuttleworth TL, Wilson MD, Wicklow BA, Wilkins JA, Triggs-Raine BL (June 2002). "Characterization of the murine hyaluronidase gene region reveals complex organization and cotranscription of Hyal1 with downstream genes, Fus2 and Hyal3". The Journal of Biological Chemistry. 277 (25): 23008–18. doi:10.1074/jbc.M108991200. PMID 11929860.
- Zegerman P, Bannister AJ, Kouzarides T (January 2000). "The putative tumour suppressor Fus-2 is an N-acetyltransferase". Oncogene. 19 (1): 161–3. doi:10.1038/sj.onc.1203234. PMID 10644992.
- Yi Lo PH, Chung Leung AC, Xiong W, Law S, Duh FM, Lerman MI, Stanbridge EJ, Lung ML (March 2006). "Expression of candidate chromosome 3p21.3 tumor suppressor genes and down-regulation of BLU in some esophageal squamous cell carcinomas". Cancer Letters. 234 (2): 184–92. doi:10.1016/j.canlet.2005.03.036. PMID 15885884.
- Lerman MI, Minna JD (November 2000). "The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium". Cancer Research. 60 (21): 6116–33. PMID 11085536.
- Duh FM, Fivash M, Moody M, Li Lung M, Guo X, Stanbridge E, Dean M, Voevoda M, Hu LF, Kashuba V, Zabarovsky ER, Qian CN, Godbole S, Tean Teh B, Lerman MI (February 2004). "Characterization of a new SNP c767A/T (Arg222Trp) in the candidate TSG FUS2 on human chromosome 3p21.3: prevalence in Asian populations and analysis of association with nasopharyngeal cancer". Molecular and Cellular Probes. 18 (1): 39–44. doi:10.1016/j.mcp.2003.09.002. PMID 15036368.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.