N,N'-Di-n-butylthiourea
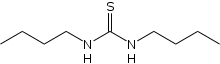
N,N′-Di-n-butylthiourea is an organic compound with the formula S=C(N(H)Bu)2 (Bu = butyl). A symmetrical N,N′-dialkyl thiourea derivative, it is a white solid. Like other thiourea derivatives, it features a planar core. The C=S bond distance is 1.712(2) Å, while C−N distances are in range of 1.33 to 1.46 Å.[1] Molecules of this compound exhibit syn-anti conformation.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N'-dibutylthiourea | |
| Other names
1,3-Dibutylthiourea 1,3-Di-n-butylthiourea Bis(butylamino)methane-1-thione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.341 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H20N2S | |
| Molar mass | 188.33 g·mol−1 |
| Appearance | White to yellowish crystals |
| Density | 1.089 g cm−3 (diffraction) |
| Melting point | 64 to 67 °C (147 to 153 °F; 337 to 340 K) |
| Structure | |
| monoclinic | |
| P21/c, No. 14 | |
a = 12.6395(6) Å, b = 10.0836(6) Å, c = 9.0128(5) Å α = 90°, β = 90.476(5)°, γ = 90° | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
N,N′-Di-n-butylthiourea can be obtained in these routes:
- Reaction of n-butylamine with carbon disulfide in the presence of alumina.[3]
- Reaction of n-butylamine and n-butylisothiocyanate.[4]
gollark: I'd like to note that you can't add stuff to the package manager for everyone to see without Kepler approving/adding it somehow. You would probably need to submit a PR somewhere. Best to actually write the program first.
gollark: <@186486131565527040> https://wiki.computercraft.cc/User:Yemmel/Project_ideas
gollark: I meant CC ones, but those work too.
gollark: https://wiki.computercraft.cc/User:Yemmel/Project_ideas
gollark: There's a good list somewhere...
References
- Okuniewski, Andrzej; Dąbrowska, Agnieszka; Chojnacki, Jarosław (2011). "1,3-Di-n-butylthiourea". Acta Crystallogr. E. 67 (4): o925. doi:10.1107/S1600536811009743. PMC 3099899. PMID 21754195.
- Sahu, Sandhyamayee; Sahoo, Prangya R.; Patel, Sabita; Mishra, B. K. (2011). "Oxidation of thiourea and substituted thioureas: a review". J. Sulfur Chem. 32 (2): 171–197. doi:10.1080/17415993.2010.550294.
- Ranu, B. C.; Dey, S. S.; Bag, S. (2003). "A simple and green procedure for the synthesis of N,N′-disubstituted thioureas on the surface of alumina under microwave irradiation". Arkivoc. 2003: 14. doi:10.3998/ark.5550190.0004.903.
- Moore, M. L.; Crossley, F. S. (1941). "Methylthiourea". Org. Synth. 21: 83. doi:10.15227/orgsyn.021.0083.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.