Moseley's law
Moseley's law is an empirical law concerning the characteristic x-rays emitted by atoms. The law had been discovered and published by the English physicist Henry Moseley in 1913-1914.[1][2] Until Moseley's work, "atomic number" was merely an element's place in the periodic table and was not known to be associated with any measurable physical quantity.[3] In brief, the law states that the square root of the frequency of the emitted x-ray is approximately proportional to the atomic number.
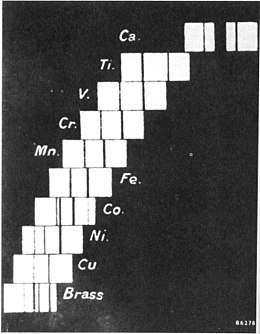
History

The historic periodic table was roughly ordered by increasing atomic weight, but in a few famous cases the physical properties of two elements suggested that the heavier ought to precede the lighter. An example is cobalt having a weight of 58.9 and nickel having an atomic weight of 58.7.
Henry Moseley and other physicists used x-ray diffraction to study the elements, and the results of their experiments led to organizing the periodic table by proton count.
Apparatus
Since the spectral emissions for the heavier elements would be in the soft X-ray range (absorbed by air), the spectrometry apparatus had to be enclosed inside a vacuum.[4] Details of the experimental setup are documented in the journal articles "The High-Frequency Spectra of the Elements" Part I[1] and Part II.[2]
Results
Moseley found that the lines (in Siegbahn notation) were indeed related to the atomic number, Z.[2]
Following Bohr's lead, Moseley found that for the spectral lines, this relationship could be approximated by a simple formula, later called Moseley's Law.
where:
- is the frequency of the observed x-ray emission line
- and are constants that depend on the type of line (that is, K, L, etc. in x-ray notation)
Rydberg frequency and = 1 for lines, and (Rydberg frequency) and = 7.4 for lines.[2]
Derivation
Moseley derived his formula empirically by line fitting the square roots of the X-ray frequencies plotted by atomic number[2], and his formula could be explained in terms of the Bohr model of the atom.
in which
- is the permittivity of free space
- is the mass of an electron
- is the charge of an electron
- is the quantum number of final energy level
- is the quantum number of initial energy level
It is assumed that the final energy level is less than the initial energy level.
Considering the empirically found constant that approximately reduced (or apparently "screened") the energy of the charges, Bohr's formula for Moseley's X-ray transitions became:
or (dividing both sides by h to convert E to ):
The coefficient in this formula simplifies to a frequency of 3/4h Ry, with an approximate value of 2.47×1015 Hz.
Screening
A simplified explanation for the effective charge of a nucleus being one less than its actual charge is that an unpaired electron in the K-shell screens it.[5][6] An elaborate discussion criticizing Moseley's interpretation of screening can be found in a paper by Whitaker[7] which is repeated in most modern texts.
A list of experimentally found X-ray transitions is available at NIST.[8] Theoretical energies can be computed to a much greater accuracy than Moseley's law using a particle physics simulation method such as Dirac-Fock.[9]
See also
- Auger electron spectroscopy, a similar phenomenon with increased x-ray yield from species of higher atomic number
References
- Moseley, Henry G. J. (1913). Smithsonian Libraries. "The High-Frequency Spectra of the Elements". The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science. 6. London-Edinburgh: London : Taylor & Francis. 26: 1024–1034.
- Moseley, Henry G. J. (1914). "The High-Frequency Spectra of the Elements. Part II". Philosophical Magazine. 6. 27: 703–713.
- e.g. Mehra, J.; Rechenberg, H. (1982). The historical development of quantum theory. Vol. 1, Part 1. New York: Springer-Verlag. pp. 193–196. ISBN 3-540-90642-8.
- Bragg, W. H. (1915). X Rays and Crystal Structure. G. Bell and Sons, Ltd. pp. 75–87.
- K. R. Naqvi (1996). "The physical (in)significance of Moseley's screening parameter". American Journal of Physics. 64 (10): 1332. Bibcode:1996AmJPh..64.1332R. doi:10.1119/1.18381.
- A. M. Lesk (1980). "Reinterpretation of Moseley's experiments relating K alpha line frequencies and atomic number". American Journal of Physics. 48 (6): 492–493. Bibcode:1980AmJPh..48..492L. doi:10.1119/1.12320.
- Whitaker, M. A. B. (1999). "The Bohr–Moseley synthesis and a simple model for atomic x-ray energies". European Journal of Physics. 20 (3): 213–220. Bibcode:1999EJPh...20..213W. doi:10.1088/0143-0807/20/3/312.
- "X-ray Transition Energies Database".
- "Theoretical Transition Energies". X-ray Transition Energies Database.
External links
- Oxford Physics Teaching - History Archive, "Exhibit 12 - Moseley's graph" (Reproduction of the original Moseley diagram showing the square root frequency dependence)