McLafferty rearrangement
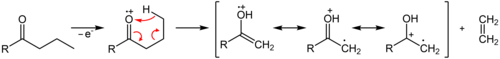
The McLafferty rearrangement is a reaction observed in mass spectrometry during the fragmentation or dissociation of organic molecules. It is sometimes found that a molecule containing a keto-group undergoes β-cleavage, with the gain of the γ-hydrogen atom, as first reported by Nicholson working in the Division of Chemical Physics at the CSIRO in Australia.[1] This rearrangement may take place by a radical or ionic mechanism.
The reaction
A description of the reaction was later published by the American chemist Fred McLafferty in 1959 leading to his name being associated with the process.[2][3][4]
gollark: Sailing as a date is probably bad because if it becomes awkward it would take some time to return to shore or whatever.
gollark: Do NOT believe them.
gollark: They are clearly out to steal your boat.
gollark: It's COVID-22 (the prerelease).
gollark: Apioforms?
See also
References
- A. J. C. Nicholson (1954). "The photochemical decomposition of the aliphatic methyl ketones". Trans. Faraday Soc. 50: 1067–1073. doi:10.1039/TF9545001067.
- F. W. McLafferty (1959). "Mass Spectrometric Analysis. Molecular Rearrangements". Anal. Chem. 31 (1): 82–87. doi:10.1021/ac60145a015.
- Gross ML (2004). "Focus in honor of Fred McLafferty, 2003 Distinguished Contribution awardee, for the discovery of the "McLafferty Rearrangement"". J. Am. Soc. Mass Spectrom. 15 (7): 951–5. doi:10.1016/j.jasms.2004.05.009. PMID 15234352.
- Nibbering NM (2004). "The McLafferty rearrangement: a personal recollection". J. Am. Soc. Mass Spectrom. 15 (7): 956–8. doi:10.1016/j.jasms.2004.04.025. PMID 15234353.
Further reading
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "McLafferty rearrangement". doi:10.1351/goldbook.M03772
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.