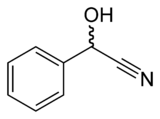
Mandelonitrile
In organic chemistry, mandelonitrile is the nitrile of mandelic acid, or the cyanohydrin derivative of benzaldehyde. Small amounts of mandelonitrile occur in the pits of some fruits.
 | |
-Mandelonitrile-3D-balls.png) | |
| Names | |
|---|---|
| IUPAC name
2-Hydroxy-2-phenylacetonitrile | |
| Other names
α-Hydroxybenzeneacetonitrile | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.758 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H7NO | |
| Molar mass | 133.150 g·mol−1 |
| Density | 1.117 g/mL |
| Melting point | −10 °C (14 °F; 263 K) (R/S)[2] |
| Boiling point | 170 °C (338 °F; 443 K) Decomposes[2] |
| Hazards | |
| Main hazards | toxic |
| R-phrases (outdated) | R23/24/25 R36/37/38 R41 |
| S-phrases (outdated) | S22 S26 S36/37/39 S45 |
| Flash point | 113 °C (235 °F; 386 K) |
| Related compounds | |
Related compounds |
mandelic acid, phenylacetonitrile |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence
Mandelonitrile is the aglycone part of the cyanogenic glycosides prunasin and amygdalin.
The naturally occurring (R)-(+) enantiomer finds use as an intermediate in the preparation of optically active α-hydroxy carboxylic acids, α-hydroxy aldehydes, α-hydroxy ketones, and 2-amino alcohols.[3]
Mandelonitrile can break down into cyanide and benzaldehyde, a reaction that can be catalyzed by the enzyme mandelonitrile lyase.
Preparation
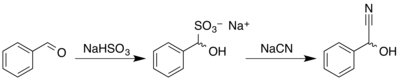
Racemic mandelonitrile may be prepared similar to many other cyanohydrins. In a one pot reaction, benzaldehyde is reacted with sodium bisulfite to give the corresponding adduct, which further reacts with aqueous sodium cyanide to give the racemic product:[4]
References
- Sigma-Aldrich product page
- The Merck Index (12th ed.). 1996.
- Kruse, C.G. In Collins, A.N. Sheldrake, G.N. Crosby, J., Eds. Chirality in Industry Chichester, UK , (1992), 279
- Corson, B. B.; Dodge, R. A.; Harris, S. A.; Yeaw, J. S. (1941). "Mandelic Acid". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 1, p. 336