Magnesium anthracene
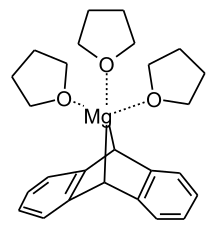
Magnesium anthracene is an organomagnesium compound that is almost invariably isolated as its adduct with three tetrahydrofuran (thf) ligands. With the formula Mg(C14H10)(thf)3, this air- and water-sensitive orange solid is obtained by heating a suspension of magnesium in a thf solution of anthracene.[1]
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.210.465 |
PubChem CID |
|
| |
| |
| Properties | |
| C26H34MgO3 | |
| Molar mass | 418.860 g·mol−1 |
| Appearance | orange solid |
| Density | 1.184 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and reactivity
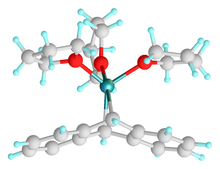
According to X-ray crystallography, the Mg center is 5-coordinate, occupying a C2O3 ligand sphere. The fold angle between the two benzo groups is 72.6°.[2]
The compound behaves as a source of the carbanion [C14H10]2- as well as a source of highly reactive Mg. With electrophiles, the compound reacts to give dihydroanthracene derivatives C14H10E2. Electrophiles include ketones, CO2, organotin chlorides, and organoaluminium chlorides. Ethylene inserts into one Mg-C bond. Hydrogen induces release of anthracene, yielding magnesium hydride (MgH2).[1]
References
- Borislav Bogdanovic (1988). "Magnesium Anthracene Systems and Their Application in Synthesis and Catalysis". Accounts of Chemical Research. 21 (7): 261–267. doi:10.1021/ar00151a002.
- L. M. Engelhardt, S. Harvey, C. L. Raston, A. H. White (1988). "Organomagnesium Reagents: The Crystal Structures of [Mg(anthracene)(THF)3] and [Mg(triphenylmethyl)Br(OEt2)2]". Journal of Organometallic Chemistry. 341 (1–3): 39. doi:10.1016/0022-328X(88)89061-2.CS1 maint: uses authors parameter (link)