Mabinlin
Mabinlins are sweet-tasting proteins extracted from the seed of mabinlang (Capparis masaikai Levl.), a Chinese plant growing in Yunnan province. There are four homologues. Mabinlin-2 was first isolated in 1983[1] and characterised in 1993,[2] and is the most extensively studied of the four. The other variants of mabinlin-1, -3 and -4 were discovered and characterised in 1994.[3]
| Mabinlin 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | ? | ||||||
| Symbol | 2SS1_CAPMA | ||||||
| UniProt | P80351 | ||||||
| |||||||
| Mabinlin 2 | |||||||
|---|---|---|---|---|---|---|---|
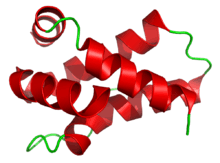 Also known as: Mabinlin II, MAB II | |||||||
| Identifiers | |||||||
| Organism | ? | ||||||
| Symbol | 2SS2_CAPMA | ||||||
| PDB | 2DS2 | ||||||
| UniProt | P30233 | ||||||
| |||||||
| Mabinlin 3 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | ? | ||||||
| Symbol | 2SS3_CAPMA | ||||||
| UniProt | P80352 | ||||||
| |||||||
| Mabinlin 4 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | ? | ||||||
| Symbol | 2SS4_CAPMA | ||||||
| UniProt | P80353 | ||||||
| |||||||
Protein structures
The 4 mabinlins are very similar in their amino acids sequences (see below).
Chain A
M-1: EPLCRRQFQQ HQHLRACQRY IRRRAQRGGL VD
M-2: QLWRCQRQFL QHQRLRACQR FIHRRAQFGG QPD
M-3: EPLCRRQFQQ HQHLRACQRY LRRRAQRGGL AD
M-4: EPLCRRQFQQ HQHLRACQRY LRRRAQRG
Chain B
M-1: EQRGPALRLC CNQLRQVNKP CVCPVLRQAA HQQLYQGQIE GPRQVRQLFR AARNLPNICK IPAVGRCQFT RW
M-2: QPRRPALRQC CNQLRQVDRP CVCPVLRQAA QQVLQRQIIQ GPQQLRRLFD AARNLPNICN IPNIGACPFR AW
M-3: EQRGPALRLC CNQLRQVNKP CVCPVLRQAA HQQLYQGQIE GPRQVRRLFR AARNLPNICK IPAVGRCQFT RW
M-4: EQRGPALRLC CNQLRQVNKP CVCPVLRQAA HQQLYQGQIE GPRQVRRLFR AARNLPNICK IPAVGRCQFT RW
Amino acid sequence of Mabinlins homologues are adapted from Swiss-Prot biological database of protein.[4][5][6][7]
The molecular weights of Mabinlin-1, Mabinlin-3 and Mabinlin-4 are 12.3 kDa, 12.3 kDa and 11.9 kDa, respectively.[3]
With a molecular weight of 10.4kDa, mabinlin-2 is lighter than mabinlin-1. It is a heterodimer consisting of two different chains A and B produced by post-translational cleavage. The A chain is composed of 33 amino acid residues and the B chain is composed of 72 amino acid residues. The B chain contains two intramolecular disulfide bonds and is connected to the A chain through two intermolecular disulfide bridges.[2][8]
Mabinlin-2 is the sweet-tasting protein with the highest known thermostability,[9] which is due to the presence of the four disulfide bridges.[10] It has been suggested also that the difference in the heat stability of the different mabinlin homologues is due to the presence of an arginine residue (heat-stable homologue) or a glutamine (heat-unstable homologue) at position 47 in the B-chain.[3]
The sequences of Mabilins cluster with Napins (InterPro: IPR000617).
Sweetness properties
Mabinlins sweetness were estimated to be about 100-400 times that of sucrose on molar basis, 10 times sucrose on a weight basis,[2][3] which make them less sweet than thaumatin (3000 times) but elicit a similar sweetness profile.[11]
The sweetness of mabinlin-2 is unchanged after 48 hours incubation at 80 °C.[2]
Mabinlin-3 and -4 sweetness stayed unchanged after 1 hour at 80 °C, while mabinlin-1 loses sweetness after 1 hour at the same condition.[3][12]
As a sweetener
Mabinlins, as proteins, are readily soluble in water and found to be highly sweet; however, mabinlin-2 with its high heat stability has the best chance to be used as a sweetener.
During the past decade, attempts have been made to produce mabinlin-2 industrially. The sweet-tasting protein has been successfully synthesised by a stepwise solid-phase method in 1998, however the synthetic protein had an astringent-sweet taste.[8]
Mabinlin-2 has been expressed in transgenic potato tubers, but no explicit results have been reported yet.[13] However, patents to protect production of recombinant mabinlin by cloning and DNA sequencing have been issued.[14]
References
- Hu Z, He M (1983). "Studies on mabinlin, a sweet protein from the seeds of Capparis masaikai levl. I. extraction, purification and certain characteristics". Acta Botan. Yunnan. (5): 207–212.
- Liu X, Maeda S, Hu Z, Aiuchi T, Nakaya K, Kurihara Y (January 1993). "Purification, complete amino acid sequence and structural characterization of the heat-stable sweet protein, mabinlin II". European Journal of Biochemistry. 211 (1–2): 281–7. doi:10.1111/j.1432-1033.1993.tb19896.x. PMID 8425538.
- Nirasawa S, Nishino T, Katahira M, Uesugi S, Hu Z, Kurihara Y (August 1994). "Structures of heat-stable and unstable homologues of the sweet protein mabinlin. The difference in the heat stability is due to replacement of a single amino acid residue". European Journal of Biochemistry. 223 (3): 989–95. doi:10.1111/j.1432-1033.1994.tb19077.x. PMID 8055976.
- Universal protein resource accession number P80351 for "Sweet protein mabinlin-1" at UniProt.
- Universal protein resource accession number P30233 for "Sweet protein mabinlin-2" at UniProt.
- Universal protein resource accession number P80352 for "Sweet protein mabinlin-3" at UniProt.
- Universal protein resource accession number P80353 for "Sweet protein mabinlin-4" at UniProt.
- Kohmura M, Ariyoshi Y (October 1998). "Chemical synthesis and characterization of the sweet protein mabinlin II". Biopolymers. 46 (4): 215–23. doi:10.1002/(SICI)1097-0282(19981005)46:4<215::AID-BIP3>3.0.CO;2-S. PMID 9715665.
- Guan RJ, Zheng JM, Hu Z, Wang DC (July 2000). "Crystallization and preliminary X-ray analysis of the thermostable sweet protein mabinlin II". Acta Crystallographica Section D. 56 (Pt 7): 918–9. doi:10.1107/S0907444900005850. PMID 10930844.
- Nirasawa S, Liu X, Nishino T, Kurihara Y (October 1993). "Disulfide bridge structure of the heat-stable sweet protein mabinlin II". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1202 (2): 277–80. doi:10.1016/0167-4838(93)90016-K. PMID 8399391.
- Kurihara Y (1992). "Characteristics of antisweet substances, sweet proteins, and sweetness-inducing proteins". Critical Reviews in Food Science and Nutrition. 32 (3): 231–52. doi:10.1080/10408399209527598. PMID 1418601.
- Kurihara Y, Nirasawa S (1997). "Structures and activities of sweetness-inducing substances (miraculin, curculin, strogin) and the heat-stable sweet protein, mabinlin" (PDF). Foods and Food Ingredients Journal of Japan (174): 67–74. Archived from the original (PDF) on 2013-09-12. Retrieved 2007-10-01.
- Xiong LW, Sun S (1996). "Molecular cloning and transgenic expression of the sweet protein mabinlin in potato tubers". Plant Physiology. 111 (2): 147.
- US granted 6051758, Sun S, Xiong L, Hu Z, Chen H, "Recombinant Sweet protein Mabinlin", issued 18 April 2000, assigned to University of Hawaii
External links
