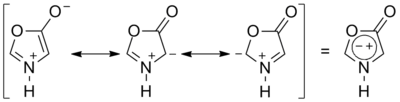
Münchnone
Münchnone (synonyms: 1,3-oxazolium-5-oxide; 1,3-oxazolium-5-olate; anhydro-5-hydroxy-1,3-oxazolium hydroxide; 5-hydroxy-1,3-oxazolium hydroxide, inner salt; oxido-oxazolium) is a mesoionic heterocyclic aromatic chemical compound.
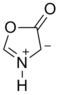
Synthesis and reactivity
The first preparation of a münchnone derivative was reported in 1958 by Lawson & Miles by cyclodehydration of 2-pyridone-N-acetic acid with acetic anhydride.[1] The azomethine ylide reactivity of münchnones, and their reaction with alkynes in the synthesis of pyrroles, was first published by Huisgen et al.[2][3] The Huisgen group followed up with a thorough investigation of the chemical properties, reactivity, and utility of münchnones towards the synthesis of many other products.[4][5] As such, they are typically credited for the discovery of the münchnone class of molecules. While certain substituted münchnones are stable and easily isolated under ambient conditions, the majority are unstable, including the parent münchnone itself. Münchnones are typically used as 1,3-dipolar cycloaddition substrates in the synthesis of pyrroles by their in situ generation in the presence of alkynes.
References
- Lawson, A.; Miles, D. H. Chem. Ind. (London) 1958, 461.
- Huisgen, R.; Gotthardt, H.; Bayer, H. O. Angew. Chem. Int. Ed. Engl. 1964, 3, 135. (doi:10.1002/anie.196401353)
- Huisgen, R.; Gotthardt, H.; Bayer, H. O.; Schaefer, F.C. Angew. Chem. Int. Ed. Engl. 1964, 3, 136. (doi:10.1002/anie.196401361)
- Gingrich, H. L.; Baum, J. S. In Oxazoles, Chemistry of Heterocyclic Compounds; Turchi, I. J., Ed.; Wiley: New York, 1986; Vol. 45. (doi:10.1002/9780470187289.ch4)
- Gribble, G. W. In Oxazoles: Synthesis, Reactions, and Spectroscopy, A; Palmer, D. C., Ed.; Wiley: New York, 2003; Vol. 60. (doi:10.1002/0471428035.ch4)