Leveling effect
Leveling effect or solvent leveling refers to the effect of solvent on the properties of acids and bases. The strength of a strong acid is limited ("leveled") by the basicity of the solvent. Similarly the strength of a strong base is leveled by the acidity of the solvent. When a strong acid is dissolved in water, it reacts with it to form hydronium ion (H3O+).[2] An example of this would be the following reaction, where "HA" is the strong acid:
- HA + H2O → A− + H3O+
Any acid that is stronger than H3O+ reacts with H2O to form H3O+. Therefore, no acid stronger than H3O+ exists in H2O. For example, aqueous perchloric acid (HClO4), aqueous hydrochloric acid (HCl) and aqueous nitric acid (HNO3) are all completely ionized, and are all equally strong acids.[3]
Similarly, when ammonia is the solvent, the strongest acid is ammonium (NH4+), thus HCl and a super acid exert the same acidifying effect.
The same argument applies to bases. In water, OH− is the strongest base. Thus, even though sodium amide (NaNH2) is an exceptional base (pKa of NH3 ~ 33), in water it is only as good as sodium hydroxide. On the other hand, NaNH2 is a far more basic reagent in ammonia than is NaOH.
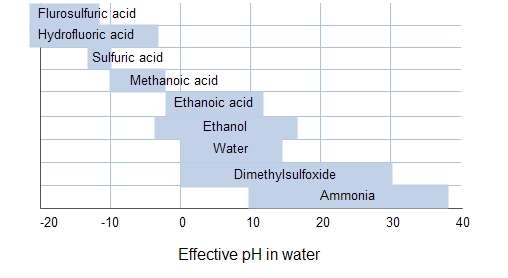
The pH range allowed by a particular solvent is called the acid-base discrimination window.[1]
Leveling and differentiating solvents
Strong bases are leveling solvents for acids, weak bases are differentiating solvents for acids. In a leveling solvent, many acids are completely dissociated and are thus of the same strength. All acids tend to become indistinguishable in strength when dissolved in strongly basic solvents owing to the greater affinity of strong bases for protons. This is called the leveling effect.
In a differentiating solvent on the other hand, various acids dissociate to different degrees and thus have different strengths. For example, anhydrous acetic acid (CH3COOH) as solvent is a weaker proton acceptor than water. Strong aqueous acids such as hydrochloric acid and perchloric acid are only partly dissociated in anhydrous acetic acid and their strengths are unequal; in fact perchloric acid is about 5000 times stronger than hydrochloric acid in this solvent.[3] A weakly basic solvent such as acetic acid has less tendency than a more strongly basic one such as water to accept a proton. Similarly a weakly acidic solvent has less tendency to donate protons than a strong acid.
Because of the leveling effect of common solvents, studies on super acids are conducted in more differentiating solvents that are very weakly basic such as sulfur dioxide (liquefied) and SO2ClF.[4]
Types of solvent on the basis of proton interaction
On the basis of proton interaction, solvents are of four types,
(i) Protophilic solvents: Solvents which have greater tendency to accept protons, i.e., water, alcohol, liquid ammonia, etc.
(ii) Protogenic solvents: Solvents which have the tendency to produce protons, i.e., water, liquid hydrogen chloride, glacial acetic acid, etc.
(iii) Amphiprotic solvents: Solvents which act both as protophilic or protogenic, e.g., water, ammonia, ethyl alcohol, etc.
(iv) Aprotic solvents: Solvents which neither donate nor accept protons, e.g., benzene, carbon tetrachloride, carbon disulphide, etc.
HCl acts as an acid in H2O, a stronger acid in NH3, a weak acid in CH3COOH, neutral in C6H6 and a weak base in HF.
References
- Atkins, P.W. (2010). Shriver and Atkins' Inorganic Chemistry, Fifth Edition. Oxford University Press. pp. 121. ISBN 978-1-42-921820-7.
- Zumdahl, S. S. “Chemistry” Heath, 1986: Lexington, MA. ISBN 0-669--04529-2.
- Skoog, Douglas A.; West, Donald M.; Holler, F. James; Crouch, Stanley R. (2014). Fundamentals of Analytical Chemistry (9th ed.). Brooks/Cole. pp. 201–202. ISBN 978-0-495-55828-6.
- Olah, G. A.; Prakash, G. K. S.; Wang, Q.; Li, X. (2001). "Hydrogen Fluoride-Antimony(V) Fluoride". In Paquette, L. (ed.). Hydrogen Fluoride–Antimony(V) Fluoride. Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rh037m. ISBN 978-0471936237.