LYPLAL1
Lysophospholipase-like 1 is a protein in humans that is encoded by the LYPLAL1 gene. [1] The protein is a α/β-hydrolase of uncharacterized metabolic function. Genome-wide association studies in humans have linked the gene to fat distribution[2] and waist-to-hip ratio.[3] The protein's enzymatic function is unclear. LYPLAL1 was reported to act as a triglyceride lipase in adipose tissue[4] and another study suggested that the protein may play a role in the depalmitoylation of calcium-activated potassium channels.[5] However, LYPLAL1 does not depalmitoylate the oncogene Ras[6] and a structural and enzymatic study concluded that LYPLAL1 is generally unable to act as a lipase and is instead an esterase that prefers short-chain substrates, such as acetyl groups.[7] Structural comparisons have suggested that LYPLAL1 might be a protein deacetylase, but this has not been experimentally tested.[8]
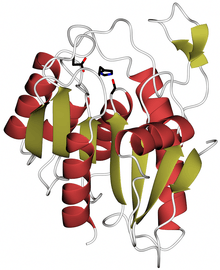 Crystal structure of human LYPLAL1, PDB code 3u0v. Alpha helices are in red, beta strands in gold, catalytic site residues in black. | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Lysophospholipase-like protein 1 | ||||||||
| Pfam | PF02230 | ||||||||
| InterPro | IPR029058 | ||||||||
| CATH | 3u0v | ||||||||
| SCOPe | 3u0v / SUPFAM | ||||||||
| |||||||||
Relationship to acyl-protein thioesterases
Sequence conservation and structural homology suggest a close relationship of LYPLAL1 proteins to acyl-protein thioesterases, and, therefore, it has been suggested that LYPLAL1 might be the third human acyl-protein thioesterase.[9] However, the major structural difference between both protein families has been established in the hydrophobic substrate binding tunnel, which has been identified in human acyl-protein thioesterases 1[10] and 2,[11] as well as in Zea mays acyl-protein thioesterase 2.[12] In LYPLAL1, this tunnel is closed due to a different loop conformation, changing the enzyme's substrate specificity to short acyl chains.[7]
Model organisms
Model organisms have been used in the study of LYPLAL1 function. A conditional knockout mouse line called Lyplal1tm1a(KOMP)Wtsi was generated at the Wellcome Trust Sanger Institute.[13] Male and female animals underwent a standardized phenotypic screen[14] to determine the effects of deletion.[15][16][17][18] Additional screens performed: - In-depth immunological phenotyping[19]
| Characteristic | Phenotype |
|---|---|
| All data available at.[14][19] | |
| Insulin | Normal |
| Homozygous viability at P14 | Normal |
| Homozygous Fertility | Normal |
| Body weight | Normal |
| Neurological assessment | Normal |
| Grip strength | Normal |
| Dysmorphology | Normal |
| Indirect calorimetry | Normal |
| Glucose tolerance test | Normal |
| Auditory brainstem response | Normal |
| DEXA | Normal |
| Radiography | Normal |
| Eye morphology | Normal |
| Clinical chemistry | Normal |
| Haematology 16 Weeks | Normal |
| Peripheral blood leukocytes 16 Weeks | Normal |
| Heart weight | Normal |
| Salmonella infection | Normal |
| Epidermal Immune Composition | Normal |
References
- "Entrez Gene: Lysophospholipase-like 1". Retrieved 2013-02-27.
- Benjamin AM, Suchindran S, Pearce K, Rowell J, Lien LF, Guyton JR, McCarthy JJ (2011). "Gene by sex interaction for measures of obesity in the framingham heart study". Journal of Obesity. 2011: 329038. doi:10.1155/2011/329038. PMC 3021872. PMID 21253498.
- Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, et al. (November 2010). "Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution". Nature Genetics. 42 (11): 949–60. doi:10.1038/ng.685. PMC 3000924. PMID 20935629.
- Steinberg GR, Kemp BE, Watt MJ (October 2007). "Adipocyte triglyceride lipase expression in human obesity". American Journal of Physiology. Endocrinology and Metabolism. 293 (4): E958-64. doi:10.1152/ajpendo.00235.2007. PMID 17609260.
- Tian L, McClafferty H, Knaus HG, Ruth P, Shipston MJ (April 2012). "Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels". The Journal of Biological Chemistry. 287 (18): 14718–25. doi:10.1074/jbc.M111.335547. PMC 3340283. PMID 22399288.
- Görmer K, Bürger M, Kruijtzer JA, Vetter I, Vartak N, Brunsveld L, Bastiaens PI, Liskamp RM, Triola G, Waldmann H (May 2012). "Chemical-biological exploration of the limits of the Ras de- and repalmitoylating machinery". ChemBioChem. 13 (7): 1017–23. doi:10.1002/cbic.201200078. PMID 22488913.
- Bürger M, Zimmermann TJ, Kondoh Y, Stege P, Watanabe N, Osada H, Waldmann H, Vetter IR (January 2012). "Crystal structure of the predicted phospholipase LYPLAL1 reveals unexpected functional plasticity despite close relationship to acyl protein thioesterases". Journal of Lipid Research. 53 (1): 43–50. doi:10.1194/jlr.M019851. PMC 3243480. PMID 22052940.
- Bürger M, Chory J (2018). "Structural and chemical biology of deacetylases for carbohydrates, proteins, small molecules and histones". Communications Biology. 1: 217. doi:10.1038/s42003-018-0214-4. PMC 6281622. PMID 30534609.
- Zeidman R, Jackson CS, Magee AI (January 2009). "Protein acyl thioesterases (Review)". Molecular Membrane Biology. 26 (1): 32–41. doi:10.1080/09687680802629329. hdl:10044/1/1452. PMID 19115143.
- Devedjiev Y, Dauter Z, Kuznetsov SR, Jones TL, Derewenda ZS (November 2000). "Crystal structure of the human acyl protein thioesterase I from a single X-ray data set to 1.5 A". Structure. 8 (11): 1137–46. doi:10.1016/s0969-2126(00)00529-3. PMID 11080636.
- Won SJ, Davda D, Labby KJ, Hwang SY, Pricer R, Majmudar JD, Armacost KA, Rodriguez LA, Rodriguez CL, Chong FS, Torossian KA, Palakurthi J, Hur ES, Meagher JL, Brooks CL, Stuckey JA, Martin BR (December 2016). "Molecular Mechanism for Isoform-Selective Inhibition of Acyl Protein Thioesterases 1 and 2 (APT1 and APT2)". ACS Chemical Biology. 11 (12): 3374–3382. doi:10.1021/acschembio.6b00720. PMC 5359770. PMID 27748579.
- Bürger M, Willige BC, Chory J (December 2017). "A hydrophobic anchor mechanism defines a deacetylase family that suppresses host response against YopJ effectors". Nature Communications. 8 (1): 2201. doi:10.1038/s41467-017-02347-w. PMC 5736716. PMID 29259199.
- Gerdin AK (2010). "The Sanger Mouse Genetics Programme: high throughput characterisation of knockout mice". Acta Ophthalmologica. 88: 925–7. doi:10.1111/j.1755-3768.2010.4142.x.
- "International Mouse Phenotyping Consortium".
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A (June 2011). "A conditional knockout resource for the genome-wide study of mouse gene function". Nature. 474 (7351): 337–42. doi:10.1038/nature10163. PMC 3572410. PMID 21677750.
- Dolgin E (June 2011). "Mouse library set to be knockout". Nature. 474 (7351): 262–3. doi:10.1038/474262a. PMID 21677718.
- Collins FS, Rossant J, Wurst W (January 2007). "A mouse for all reasons". Cell. 128 (1): 9–13. doi:10.1016/j.cell.2006.12.018. PMID 17218247.
- White JK, Gerdin AK, Karp NA, Ryder E, Buljan M, Bussell JN, et al. (July 2013). "Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes". Cell. 154 (2): 452–64. doi:10.1016/j.cell.2013.06.022. PMC 3717207. PMID 23870131.
- "Infection and Immunity Immunophenotyping (3i) Consortium".