LY294002
LY294002 is a morpholine-containing chemical compound that is a potent inhibitor of numerous proteins, and a strong inhibitor of phosphoinositide 3-kinases (PI3Ks).[1] It is generally considered a non-selective research tool, and should not be used for experiments aiming to target PI3K uniquely.[2]
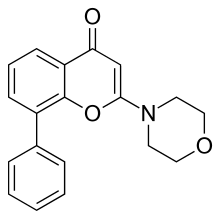 | |
| Names | |
|---|---|
| IUPAC name
2-Morpholin-4-yl-8-phenylchromen-4-one | |
| Other names
2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H17NO3 | |
| Molar mass | 307.349 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Two of these are the proto-oncogene serine/threonine-protein kinase (PIM1) and the phosphatidylinositol-4,5-bisphosphate 3-kinase P110 gamma|catalytic subunit gamma isoform.[3] With an IC50 of 1.4 μM it is somewhat less potent than wortmannin, another well-known PI3 kinase inhibitor. However, LY294002 is a reversible inhibitor of PI3K whereas wortmannin acts irreversibly.[4]
Application of LY294002 causes a substantial acceleration of MEPP frequency (150 μM) at the frog neuromuscular junction through a mechanism that is independent of intraterminal calcium. LY294002 causes the release of MEPPs through a perturbation of synaptotagmin function.[5]
LY294002 is also a BET inhibitor (e.g. of BRD2, BRD3, and BRD4).[6]
Application
Research
It has been shown that LY294002 administration has an additive effect on quercetin antiviral activity against hepatitis C virus.[7]
References
- Maira; et al. (2009). "PI3K inhibitors for cancer treatment: where do we stand?". Biochemical Society Transactions. 37: 265–272. doi:10.1042/BST0370265. PMID 19143644.
- Arrowsmith, Cheryl H.; Audia, James E.; Austin, Christopher; Baell, Jonathan; Bennett, Jonathan; Blagg, Julian; Bountra, Chas; Brennan, Paul E.; Brown, Peter J. (2015-08-01). "The promise and peril of chemical probes". Nature Chemical Biology. 11 (8): 536–541. doi:10.1038/nchembio.1867. ISSN 1552-4450. PMC 4706458. PMID 26196764.
- "Card for LY294002 in DrugBank". DrugBank. Retrieved 2009-09-25.
- Chris J. Vlahos; et al. (1994). "A Specific Inhibitor of Phosphatidylinositol 3-Kinase, 2-(4-Morpholinyl)-8-phenyl-4H-l-benzopyran-4-one (LY294002)". Journal of Biological Chemistry. 269 (7): 5241–5248. PMID 8106507.
- Searl TJ, Silinsky EM (Dec 2005). "LY 294002 inhibits adenosine receptor activation by a mechanism independent of effects on PI-3 kinase or casein kinase II". Purinergic Signal. 1 (4): 389–94. doi:10.1007/s11302-005-0778-6. PMC 2096559. PMID 18404524.
- Dittmann, Antje; Werner, Thilo; Chung, Chun-Wa; Savitski, Mikhail M.; Fälth Savitski, Maria; Grandi, Paola; Hopf, Carsten; Lindon, Matthew; Neubauer, Gitte; Prinjha, Rabinder K.; Bantscheff, Marcus; Drewes, Gerard (2013). "The Commonly Used PI3-Kinase Probe LY294002 is an Inhibitor of BET Bromodomains". ACS Chemical Biology. 9 (2): 495–502. doi:10.1021/cb400789e. PMID 24533473.
- Pisonero-Vaquero S (Mar 2014). "Modulation of PI3K-LXRα-dependent lipogenesis mediated by oxidative/nitrosative stress contributes to inhibition of HCV replication by quercetin". Lab. Invest. 94 (3): 262–274. doi:10.1038/labinvest.2013.156. PMID 24492281.