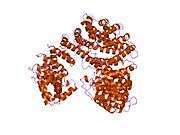
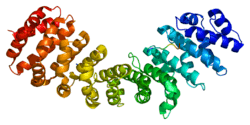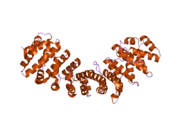Karyopherin alpha 2
Importin subunit alpha-2 is a protein that in humans is encoded by the KPNA2 gene.[5][6]
The import of proteins into the nucleus is a process that involves at least 2 steps. The first is an energy-independent docking of the protein to the nuclear envelope and the second is an energy-dependent translocation through the nuclear pore complex. Imported proteins require a nuclear localization sequence (NLS) which generally consists of a short region of basic amino acids or 2 such regions spaced about 10 amino acids apart. Proteins involved in the first step of nuclear import are members of the alpha importin family of karyopherins such as importin subunit alpha-2. These include the Xenopus protein importin and its yeast homolog, SRP1 (a suppressor of certain temperature-sensitive mutations of RNA polymerase I in Saccharomyces cerevisiae), which bind to the NLS. KPNA2 protein interacts with the NLSs of DNA helicase Q1 and SV40 T antigen and may be involved in the nuclear transport of proteins. KPNA2 also may play a role in V(D)J recombination[7]
Interactions
Karyopherin alpha 2 has been shown to interact with:
References
- GRCh38: Ensembl release 89: ENSG00000182481 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000018362 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Cuomo CA, Kirch SA, Gyuris J, Brent R, Oettinger MA (Jun 1994). "Rch1, a protein that specifically interacts with the RAG-1 recombination-activating protein". Proceedings of the National Academy of Sciences of the United States of America. 91 (13): 6156–60. doi:10.1073/pnas.91.13.6156. PMC 44157. PMID 8016130.
- Weis K, Mattaj IW, Lamond AI (May 1995). "Identification of hSRP1 alpha as a functional receptor for nuclear localization sequences". Science. 268 (5213): 1049–53. Bibcode:1995Sci...268.1049W. doi:10.1126/science.7754385. PMID 7754385.
- "Entrez Gene: KPNA2 karyopherin alpha 2 (RAG cohort 1, importin alpha 1)".
- Lin CY, Huang PH, Liao WL, Cheng HJ, Huang CF, Kuo JC, Patton WA, Massenburg D, Moss J, Lee FJ (Dec 2000). "ARL4, an ARF-like protein that is developmentally regulated and localized to nuclei and nucleoli". The Journal of Biological Chemistry. 275 (48): 37815–23. doi:10.1074/jbc.M002470200. PMID 10980193.
- Perez-Villar JJ, O'Day K, Hewgill DH, Nadler SG, Kanner SB (Oct 2001). "Nuclear localization of the tyrosine kinase Itk and interaction of its SH3 domain with karyopherin alpha (Rch1alpha)". International Immunology. 13 (10): 1265–74. doi:10.1093/intimm/13.10.1265. PMID 11581171.
- Nagoshi E, Yoneda Y (Apr 2001). "Dimerization of sterol regulatory element-binding protein 2 via the helix-loop-helix-leucine zipper domain is a prerequisite for its nuclear localization mediated by importin beta". Molecular and Cellular Biology. 21 (8): 2779–89. doi:10.1128/MCB.21.8.2779-2789.2001. PMC 86908. PMID 11283257.
- Köhler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Görlich D, Hartmann E (Nov 1999). "Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import". Molecular and Cellular Biology. 19 (11): 7782–91. doi:10.1128/mcb.19.11.7782. PMC 84838. PMID 10523667.
- Kutay U, Bischoff FR, Kostka S, Kraft R, Görlich D (Sep 1997). "Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor". Cell. 90 (6): 1061–71. doi:10.1016/s0092-8674(00)80372-4. PMID 9323134.
- Braem CV, Kas K, Meyen E, Debiec-Rychter M, Van De Ven WJ, Voz ML (May 2002). "Identification of a karyopherin alpha 2 recognition site in PLAG1, which functions as a nuclear localization signal". The Journal of Biological Chemistry. 277 (22): 19673–8. doi:10.1074/jbc.M112112200. PMID 11882654.
- Seki T, Tada S, Katada T, Enomoto T (May 1997). "Cloning of a cDNA encoding a novel importin-alpha homologue, Qip1: discrimination of Qip1 and Rch1 from hSrp1 by their ability to interact with DNA helicase Q1/RecQL". Biochemical and Biophysical Research Communications. 234 (1): 48–53. doi:10.1006/bbrc.1997.6535. PMID 9168958.
- Maiyar AC, Leong ML, Firestone GL (Mar 2003). "Importin-alpha mediates the regulated nuclear targeting of serum- and glucocorticoid-inducible protein kinase (Sgk) by recognition of a nuclear localization signal in the kinase central domain". Molecular Biology of the Cell. 14 (3): 1221–39. doi:10.1091/mbc.E02-03-0170. PMC 151592. PMID 12631736.
Further reading
- Bukrinsky MI, Haffar OK (Dec 1997). "HIV-1 nuclear import: in search of a leader". Frontiers in Bioscience. 2 (4): d578-87. doi:10.2741/A213. PMID 9366553.
- Bukrinsky MI, Haffar OK (Mar 1998). "HIV-1 nuclear import: matrix protein is back on center stage, this time together with Vpr". Molecular Medicine. 4 (3): 138–43. doi:10.1007/BF03401911. PMC 2230352. PMID 9562972.
- Kino T, Pavlakis GN (Apr 2004). "Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1". DNA and Cell Biology. 23 (4): 193–205. doi:10.1089/104454904773819789. PMID 15142377.
- Teng SC, Wu KJ, Tseng SF, Wong CW, Kao L (Sep 2006). "Importin KPNA2, NBS1, DNA repair and tumorigenesis". Journal of Molecular Histology. 37 (5–7): 293–9. doi:10.1007/s10735-006-9032-y. PMID 16752129.
- Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M (Jul 1992). "Active nuclear import of human immunodeficiency virus type 1 preintegration complexes". Proceedings of the National Academy of Sciences of the United States of America. 89 (14): 6580–4. Bibcode:1992PNAS...89.6580B. doi:10.1073/pnas.89.14.6580. PMC 49545. PMID 1631159.
- Sharova N, Bukrinskaya A (Mar 1991). "p17 and p17-containing gag precursors of input human immunodeficiency virus are transported into the nuclei of infected cells". AIDS Research and Human Retroviruses. 7 (3): 303–6. doi:10.1089/aid.1991.7.303. PMID 2064827.
- Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau NR (Dec 1995). "Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr". Journal of Virology. 69 (12): 7909–16. PMC 189735. PMID 7494303.
- Küssel P, Frasch M (Aug 1995). "Yeast Srp1, a nuclear protein related to Drosophila and mouse pendulin, is required for normal migration, division, and integrity of nuclei during mitosis". Molecular & General Genetics. 248 (3): 351–63. doi:10.1007/BF02191602. PMID 7565597.
- Gallay P, Swingler S, Song J, Bushman F, Trono D (Nov 1995). "HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase". Cell. 83 (4): 569–76. doi:10.1016/0092-8674(95)90097-7. PMID 7585960.
- Moroianu J, Hijikata M, Blobel G, Radu A (Jul 1995). "Mammalian karyopherin alpha 1 beta and alpha 2 beta heterodimers: alpha 1 or alpha 2 subunit binds nuclear localization signal and beta subunit interacts with peptide repeat-containing nucleoporins". Proceedings of the National Academy of Sciences of the United States of America. 92 (14): 6532–6. Bibcode:1995PNAS...92.6532M. doi:10.1073/pnas.92.14.6532. PMC 41552. PMID 7604027.
- Freed EO, Englund G, Martin MA (Jun 1995). "Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection". Journal of Virology. 69 (6): 3949–54. doi:10.1128/JVI.69.6.3949-3954.1995. PMC 189124. PMID 7745752.
- Gallay P, Swingler S, Aiken C, Trono D (Feb 1995). "HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator". Cell. 80 (3): 379–88. doi:10.1016/0092-8674(95)90488-3. PMID 7859280.
- von Schwedler U, Kornbluth RS, Trono D (Jul 1994). "The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes". Proceedings of the National Academy of Sciences of the United States of America. 91 (15): 6992–6. Bibcode:1994PNAS...91.6992V. doi:10.1073/pnas.91.15.6992. PMC 44324. PMID 8041734.
- Heinzinger NK, Bukrinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M (Jul 1994). "The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells". Proceedings of the National Academy of Sciences of the United States of America. 91 (15): 7311–5. Bibcode:1994PNAS...91.7311H. doi:10.1073/pnas.91.15.7311. PMC 44389. PMID 8041786.
- Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M (Oct 1993). "A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells". Nature. 365 (6447): 666–9. Bibcode:1993Natur.365..666B. doi:10.1038/365666a0. PMID 8105392.
- Dubrovsky L, Ulrich P, Nuovo GJ, Manogue KR, Cerami A, Bukrinsky M (Jan 1995). "Nuclear localization signal of HIV-1 as a novel target for therapeutic intervention". Molecular Medicine. 1 (2): 217–30. doi:10.1007/BF03401569. PMC 2229944. PMID 8529100.
- Gallay P, Stitt V, Mundy C, Oettinger M, Trono D (Feb 1996). "Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import". Journal of Virology. 70 (2): 1027–32. doi:10.1128/JVI.70.2.1027-1032.1996. PMC 189908. PMID 8551560.
- Bukrinskaya AG, Ghorpade A, Heinzinger NK, Smithgall TE, Lewis RE, Stevenson M (Jan 1996). "Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA". Proceedings of the National Academy of Sciences of the United States of America. 93 (1): 367–71. Bibcode:1996PNAS...93..367B. doi:10.1073/pnas.93.1.367. PMC 40239. PMID 8552640.