Isopropylmagnesium chloride
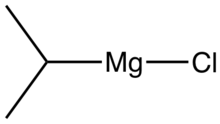
Isopropylmagnesium chloride is an organometallic compound with the general formula (CH3)2HCMgCl. This highly flammable, colorless, and moisture sensitive material is the Grignard reagent derived from isopropyl chloride. It is commercially available, usually as a solution in tetrahydrofuran. This reagent is used to prepare Grignard reagents by transmetalation reactions as well as installing isopropyl groups.[1] An illustrative generic reaction involves the generation of the Grignard reagent derived from bromo-3,5-bis(trifluoromethyl)benzene:[2]
- (CH3)2HCMgCl + (CF3)2C6H3Br → (CH3)2HCCl + (CF3)2C6H3MgBr
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.680 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H7ClMg | |
| Molar mass | 102.84 g·mol−1 |
| Solubility | Ethyl ether |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H225, H260, H314 |
| P210, P223, P231+232, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P335+334, P363, P370+378, P402+404, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isopropylmagnesium chloride is also used to prepare other isopropyl compounds, such as chlorodiisopropylphosphine:[3]
- PCl3 + 2 (CH3)2CHMgCl → [(CH3)2CH]2PCl + 2 MgCl2
This reaction exploits the bulky nature of the isopropyl substituent.
References
- Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. (2003). "Highly Functionalized Organomagnesium Reagents Prepared through Halogen–Metal Exchange". Angewandte Chemie International Edition. 42: 4302–4320. doi:10.1002/anie.200300579. PMID 14502700.CS1 maint: uses authors parameter (link)
- Johnnie L. Leazer, Jr, Raymond Cvetovich (2005). "A Practical and Safe Preparation of 3,5-Bis(trifluoromethyl)acetophenone". Org. Synth. 82: 115. doi:10.15227/orgsyn.082.0115.CS1 maint: uses authors parameter (link)
- W. Voskuil and J. F. Arens (1968). "Chlorodiisopropylphosphine". Org. Synth. 48: 47. doi:10.15227/orgsyn.048.0047.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.