Ireland–Claisen rearrangement
The Ireland–Claisen rearrangement is a chemical reaction of an allylic ester with strong base to give an γ,δ-unsaturated carboxylic acid.[1][2][3]
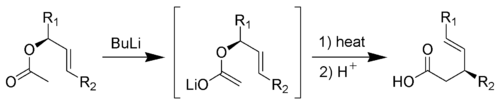
The Ireland-Claisen rearrangement
Mechanism
The Ireland–Claisen rearrangement is a type of Claisen rearrangement. The mechanism is therefore a concerted [3,3]-sigmatropic rearrangement which according to the Woodward–Hoffmann rules show a concerted, suprafacial, pericyclic reaction pathway.
gollark: I'm not sure what happens with that.
gollark: It really likes holoapioforms.
gollark: **The most gollarious response**:- make a cryoapiary-sized stack-???-???<|endoftext|>You can also do a dyson *with* a holoapiary, actually.<|endoftext|>Idea: a holoapiary-like hologram projector, which is capable of holoapioform-proofed.<|endoftext|>We should make a holoapiary-based AI.<|endoftext|>I think the holoapioapioform control system is a holoapiary, so it's not hard.<|endoftext|>https://i.redd.it/qfxjh21.jpgI don't think anyone was doing that, though.<|endoftext|>Also, it's actually a holoapioform.<|endoftext|>The holoapioform control system is a holoapiary, not a holoapiary.<|endoftext|>Well, not a holoapiary, how'd it work?<|endoftext|>I doubt it's a holoapiary, so I can't see them.<|end
gollark: I mean, I *can*, I just don't.
gollark: I will do this if I ever hook it up to something automatic and don't just have a python thing open with the model.
See also
References
- Ireland, R. E.; Mueller, R. H. (1972). "Claisen rearrangement of allyl esters". J. Am. Chem. Soc. 94 (16): 5897. doi:10.1021/ja00771a062.
- Ireland, R. E.; Mueller, R. H.; Willard, A. K. (1976). "The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation". J. Am. Chem. Soc. 98 (10): 2868–2877. doi:10.1021/ja00426a033.
- Miller, S. P.; Morken, J. P. (2004). "Catalytic Diastereoselective Reductive Claisen Rearrangement". Organic Letters. 60 (16): 2743–2745. doi:10.1021/ol026273b.
- Ziegler, F. E. (1977). "Stereo- and regiochemistry of the Claisen rearrangement: Applications to natural products synthesis". Acc. Chem. Res. 10 (6): 1423–1452. doi:10.1021/ar50114a006.
- Pereira, S.; Srebnik, M. (1993). "The Ireland–Claisen rearrangement" (PDF). Aldrichimica Acta. 26 (1): 17–29.
- Chai, Y.; Hong, S.; Lindsay, H. A.; McFarland, C.; McIntosh, M. C. (2002). "New aspects of the Ireland and related Claisen rearrangements". Tetrahedron. 58 (15): 2905–2928. doi:10.1016/S0040-4020(02)00164-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.