Iodate
An iodate is a conjugate base of iodic acid.[1] In the iodate anion, iodine is bonded to three oxygen atoms and the molecular formula is IO−
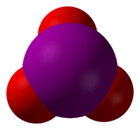
3. The molecular geometry of iodate is trigonal pyramidal.
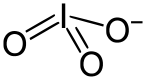
3
Iodate can be obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide.[2]
Iodates are a class of chemical compounds containing this group. Examples are sodium iodate (NaIO3), silver iodate (AgIO3), and calcium iodate (Ca(IO3)2). Iodates resemble chlorates with iodine instead of chlorine.
In acidic conditions, iodic acid is formed. Potassium hydrogen iodate (KH(IO3)2) is a double salt of potassium iodate and iodic acid and an acid as well. Iodates are used in the iodine clock reaction.
Potassium iodate, like potassium iodide, has been issued as a prophylaxis against radioiodine absorption in some countries.[3][4]
Other oxyanions
Iodine can assume oxidation states of −1, +1, +3, +5, or +7. A number of neutral iodine oxides are also known.
| Iodine oxidation state | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| Name | iodide | hypoiodite | iodite | iodate | periodate |
| Formula | I− | IO− | IO− 2 |
IO− 3 |
IO− 4 or IO5− 6 |
Natural occurrence
Minerals containing essential iodate anions are very rare. They are almost exclusively known from the caliche deposits of Chile. The deposits stand for the main source of nitrates, but iodate- and chromate-bearing minerals are also present there. The most important iodate minerals are lautarite and brüggenite, but also copper-bearing iodates (e.g., salesite) are known. In the iodate minerals iodine is always pentavalent, i.e., present as the IO−
3 anions.[5]
References
- Merriam-Webster definition
- Qiu, Chao; Sheng Han; Xingguo Cheng; Tianhui Ren (2005). "Distribution of Thioethers in Hydrotreated Transformer Base Oil by Oxidation and ICP-AES Analysis" (abstract). Industrial & Engineering Chemistry Research. 44 (11): 4151–4155. doi:10.1021/ie048833b. Retrieved 2007-05-03.
Thioethers can be oxidized to sulfoxides by periodate, and periodate is reduced to iodate
- "Archived copy". Archived from the original on 2013-10-17. Retrieved 2013-04-08.CS1 maint: archived copy as title (link)
- "Archived copy". Archived from the original on 2013-10-18. Retrieved 2013-05-22.CS1 maint: archived copy as title (link)
- http://www.mindat.org