Iminoborane
Iminoboranes comprise a group of inorganic compounds with the generic formula containing triple bonds XB≡NY, where X, Y can be H, F, RO, R2N, R3C, etc, among which the simplest form is HB≡NH. They are electronically related to acetylenes but are usually more reactive due to the polarity.[1][2][3]
Structure and Bonding
Parent compound HB≡NH
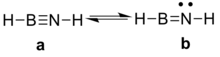
For iminoboranes, two resonance structures mainly contribute to the final structure (Figure 1).[4]
Substituent effect
Because the substituents can donate their lone pairs into one of the B-N anti-bonding orbitals, the bonding scheme can be dramatically altered when substitution occurs, and the new bonding scheme highly depends on the nature of substituents and the site of substitution, which can be investigated theoretically.[5]
HBNH
HBNH is an intermediate from the photolysis of solid H3BNH3[6][7][8] Strong shielding effect of bulky substitutions can make the iminoboranes more stable and more resistant to oligomerization, which allows for the storage at room temperature.[9]
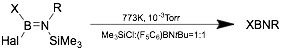
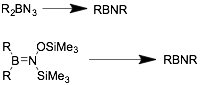
Thermal decomposition of azidoboranes and trimethyl-silyl(trimethylsilyloxy)aminoboranes can be used to access symmetric iminoboranes, especially those with bulky R groups.[10]
Reactivity
Oligomerization
Iminoboranes tend to oligomerize. Five types of oligomerization product are mainly produced: cyclodimers (1,3,2,4-diazadiboretidines, Di[11][12]), cyclotrimers (borazines, Tr), bicyclotrimers (Dewar borazines, Tr’[13]), cyclotetramers (octahydro-1,3,5,7-tetraza-2,4,6,8-tetraborocines, Te[14]), and polymers (polyiminoboranes, Po); which are shown below.[15] Which product is dominant depends on the structures of reactants and the reaction conditions. Some of the products can be interconverted.[16]
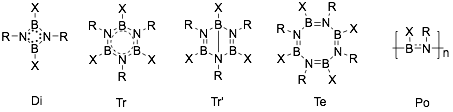
Polar Addition
Acid
If there is a π-electron donating group such as -NR2 connected to B, the neutral Lewis acids can be bonded to the N atom of the B≡N. The product shown in the following scheme is a famous diaminoboron cation [C9H18N=B=NR2]+, where the positive charge is balanced intramolecularly, not by a external anion.[17]
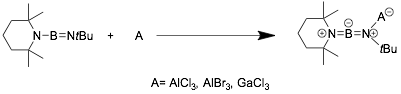
Base
The 11BNMR signals of iminoboranes can be altered when the iminoboranes are dissolved in liquid with Lewis base activity (tertiary amines, tetrahydrofuran). There may be interactions between the positive-charged B and the Lewis base, but the chemistry behind this still needs to be further investigated.[18]
Addition of protic agents
The addition of protic agents is a quantitive reaction, fast even far below 0 ℃, while the addition of protic agents of acetylenes requires higher temperature, which also indicates that iminoboranes are more reactive towards polar addition than acetylenes.[19]
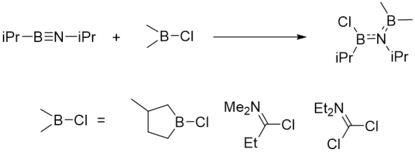
Boration
Boration reaction of iminoboranes is the addition of B-X single bond to B≡N, where -X can be -Cl (chloro-boration), -N3 (azido-boration), -SR (thio-boration), -NR2 (amino-boration) and R (alkyl-boration). One of these reactions are illustrated here.
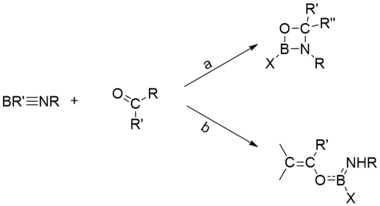
Cycloadditions
Cycloaddition is the addition of π bond to B≡N, and can be categorized into [2+2]-cycloadditions, [2+3]-cycloadditions, [2+4]-cycloadditions etc.
One of the widely investigated [2+2]-cycloadditions is the reaction of aldehydes and ketones with iminoboranes, which has two possible pathways. Usually, the relatively stable iminoboranes which are less likely to undergo the total opening of B≡N, and the ketones containing enolic protons, prefer to undergo path b.[20]
The typical [2+3]-cycloaddition is the addition of B≡N and RN3,and the typical [2+4]-cycloaddition is the addition of B≡N and cyclopentadiene.
Coordination to transition metals
Like acetylenes, iminoboranes can also coordinate with transition metals.
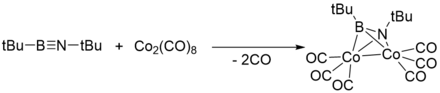
References
- Inorganic ring systems : 7th International symposium : Papers. Chivers, Tristram. Gordon and Breach Science Publishers. 1994. ISBN 978-2-88449-168-6. OCLC 81135356.CS1 maint: others (link)
- Advances in inorganic chemistry. Volume 31. Emeléus, H. J. (Harry Julius), Sharpe, A. G. New York: Academic Press. 1987. ISBN 978-0-12-023631-2. OCLC 277086713.CS1 maint: others (link)
- Paetzold, Peter (1987). Iminoboranes. Advances in Inorganic Chemistry. 31. pp. 123–170. doi:10.1016/s0898-8838(08)60223-8. ISBN 978-0-12-023631-2.
- Mó, Otilia; Yáñez, Manuel; Pendás, Angel Martín; Bene, Janet E. Del; Alkorta, Ibon; Elguero, José (2007-07-23). "Unusual substituent effects on the bonding of iminoboranes". Phys. Chem. Chem. Phys. 9 (30): 3970–3977. doi:10.1039/b702480k. hdl:10261/72489. ISSN 1463-9084. PMID 17646885.
- Mó, Otilia; Yáñez, Manuel; Pendás, Angel Martín; Bene, Janet E. Del; Alkorta, Ibon; Elguero, José (2007-07-23). "Unusual substituent effects on the bonding of iminoboranes". Phys. Chem. Chem. Phys. 9 (30): 3970–3977. doi:10.1039/b702480k. hdl:10261/72489. ISSN 1463-9084. PMID 17646885.
- Lory, Earl R.; Porter, Richard F. (1973-03-01). "Infrared studies of matrix isolated species in the hydrogen-boron-nitrogen system". Journal of the American Chemical Society. 95 (6): 1766–1770. doi:10.1021/ja00787a012. ISSN 0002-7863.
- Paetzold, Peter; Richter, Anette; Thijssen, Theo; Würtenberg, Stefan (1979-12-01). "Bildung, Struktur und Reaktivität von (Pentafluorphenyl)bor-tert-butylimid und seinem Cyclodimeren". Chemische Berichte. 112 (12): 3811–3827. doi:10.1002/cber.19791121207. ISSN 1099-0682.
- Paetzold, Peter; von Plotho, Christoph (1982-08-01). "Über weitere monomere Borimide und ihre Reaktionen". Chemische Berichte. 115 (8): 2819–2825. doi:10.1002/cber.19821150813. ISSN 1099-0682.
- Haase, Martin; Klingebiel, Uwe (1985-04-01). "Simple Synthesis of Stable Iminoboranes". Angewandte Chemie International Edition in English. 24 (4): 324. doi:10.1002/anie.198503241. ISSN 1521-3773.
- Weiß, Robert; Wagner, Klaus-Georg (1984-05-01). "Notizen. Die Erzeugung von Nitrosylsalzen in wasserfreien organischen Medien". Chemische Berichte. 117 (5): 1973–1976. doi:10.1002/cber.19841170526. ISSN 1099-0682.
- Paetzold, Peter; Eleftheriadis, Eleftherios; Minkwitz, Rolf; Wölfel, Volker; Gleiter, Rolf; Bischof, Peter; Friedrich, Gert (1988-01-01). "Bildung, Struktur und Reaktionen von Methyl(methylimino)boran". Chemische Berichte. 121 (1): 61–66. doi:10.1002/cber.19881210110. ISSN 1099-0682.
- Hess, H. (1969-11-15). "Strukturbestimmungen an Bor–Stickstoff-Verbindungen. IV. Die Kristall- und Molekularstruktur von Hexakis(trimethylsilyl)-2,4-diamino-1,3,2,4-diazadiboretidin" (PDF). Acta Crystallographica Section B (in German). 25 (11): 2342–2349. doi:10.1107/s056774086900567x. ISSN 0567-7408.
- Steuer, Holger-A.; Meller, Anton; Elter, Gernot (1985). "B-t-butyl-borazine und -diazadiboretidine". Journal of Organometallic Chemistry. 295 (1): 1–6. doi:10.1016/0022-328x(85)88065-7.
- Turner H. S. and Warne R. J. 1962 Proc. Chem. Soc. 69.
- Advances in inorganic chemistry. Volume 31. Emeléus, H. J. (Harry Julius), Sharpe, A. G. New York: Academic Press. 1987. ISBN 978-0-12-023631-2. OCLC 277086713.CS1 maint: others (link)
- Maier.G. (1978). "Tetra-tert-butyltetrahedrane". Angew. Chem. Int. Ed. Engl. 17: 520. doi:10.1002/anie.197805201.
- Nöth, Heinrich; Weber, Siegfried (1985-06-01). "Beiträge zur Chemie des Bors, 158. Addukte von Aluminium- und Galliumhalogeniden an ein Aminoiminoboran". Chemische Berichte. 118 (6): 2554–2556. doi:10.1002/cber.19851180631. ISSN 1099-0682.
- Advances in inorganic chemistry. Volume 31. Emeléus, H. J. (Harry Julius), Sharpe, A. G. New York: Academic Press. 1987. ISBN 978-0-12-023631-2. OCLC 277086713.CS1 maint: others (link)
- Nöth, Heinrich; Weber, Siegfried (1985-05-01). "Beiträge zur Chemie des Bors, 154. Addition von Trimethylsily-Verbindungen und von anderen Elektrophilen an (tert-Butylimino) (tetramethylpiperidino)boran". Chemische Berichte. 118 (5): 2144–2146. doi:10.1002/cber.19851180536. ISSN 1099-0682.
- von Kutepow, Nikolaus (1972-04-01). "Chemistry of acetylenes. Von H. G. Viehe. Marcel Dekker Inc., New York 1969. 1. Aufl., XV, 1298 S., zahlr. Tab. u. Formeln, geb. $ 59.50". Angewandte Chemie. 84 (8): 367. doi:10.1002/ange.19720840843. ISSN 1521-3757.