Pyrimidone
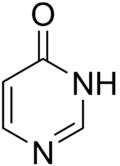
Pyrimidone is the name given to either of two heterocyclic compounds with the formula C4H4N2O: 2-pyrimidone and 4-pyrimidone. The compounds can also be called 2-hydroxypyrimidine or 4-hydroxypyrimidine respectively, based on a substituted pyrimidine, or 1,3-diazine, ring.
 | |
| Names | |
|---|---|
| IUPAC name
Pyrimidone | |
| Other names
Hydroxypyrimidine; Pyrimidinone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| Properties | |
| C4H4N2O | |
| Molar mass | 96.089 g·mol−1 |
| Appearance | White to light yellow powder |
| Melting point | 163 to 168 °C (325 to 334 °F; 436 to 441 K) |
| Hazards | |
| Main hazards | Respiratory system, eye, skin irritation |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Derivatives
Derivatives of pyrimidone are the basis of many other biological molecules, including:
- Nucleobases, such as cytosine
- Barbiturates, such as metharbital
- Antiulcer drugs including temelastine, icotidine, donetidine, and lupitidine.
gollark: Like I said, reducing the numbers just means people will spam them, like dayblooms in Botania ages ago.
gollark: Er, no.
gollark: NuclearCraft does not actually have these, nor would I want it to.
gollark: Also, what would "harder" involve? Lower output from the collectors would just induce grind.
gollark: What would you actually reasonably use it for anyway other than cooling?
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.