Hydrophilic-lipophilic balance
The hydrophilic-lipophilic balance of a surfactant is a measure of the degree to which it is hydrophilic or lipophilic, determined by calculating values for the different regions of the molecule, as described by Griffin in 1949[1] and 1954.[2] Other methods have been suggested, notably in 1957 by Davies.[3]
Griffin's method
Griffin's method for non-ionic surfactants as described in 1954 works as follows:
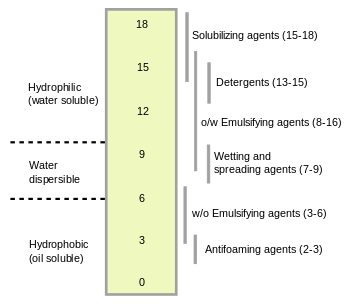
where is the molecular mass of the hydrophilic portion of the molecule, and M is the molecular mass of the whole molecule, giving a result on a scale of 0 to 20. An HLB value of 0 corresponds to a completely lipophilic/hydrophobic molecule, and a value of 20 corresponds to a completely hydrophilic/lipophobic molecule.
The HLB value can be used to predict the surfactant properties of a molecule:
Davies' method
In 1957, Davies suggested a method based on calculating a value based on the chemical groups of the molecule. The advantage of this method is that it takes into account the effect of stronger and weaker hydrophilic groups. The method works as follows:[3]
where:
- Number of hydrophilic groups in the molecule
- Value of the th hydrophilic groups (see tables)
- Number of lipophilic groups in the molecule
| Hydrophilic Groups | Group Number |
|---|---|
| -SO4−Na+ | 38.7 |
| -COO−K+ | 21.1 |
| -COO−Na+ | 19.1 |
| N (tertiary amine) | 9.4 |
| Ester (sorbitan ring) | 6.8 |
| Ester (free) | 2.4 |
| -COOH | 2.1 |
| Hydroxyl (free) | 1.9 |
| -O- | 1.3 |
| Hydroxyl (sorbitan ring) | 0.5 |
| Lipophilic Groups | Group Number |
|---|---|
| -CH- | -0.475 |
| -CH2- | -0.475 |
| CH3- | -0.475 |
| =CH- | -0.475 |
References
- Griffin, William C. (1949), "Classification of Surface-Active Agents by 'HLB'" (PDF), Journal of the Society of Cosmetic Chemists, 1 (5): 311–26, archived from the original (PDF) on 2014-08-12, retrieved 2013-05-25
- Griffin, William C. (1954), "Calculation of HLB Values of Non-Ionic Surfactants" (PDF), Journal of the Society of Cosmetic Chemists, 5 (4): 249–56, archived from the original (PDF) on 2014-08-12, retrieved 2013-05-25
- Davies JT (1957), "A quantitative kinetic theory of emulsion type, I. Physical chemistry of the emulsifying agent" (PDF), Gas/Liquid and Liquid/Liquid Interface, Proceedings of the International Congress of Surface Activity, pp. 426–38