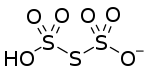
Hydrogen trithionate
Hydrogen trithionate is a partially deprotonated oxyacid (specifically as polythionic acid) can also be considered to be a partially protonated oxyanion. It can either give up a proton to become trithionate or receive one to become trithionic acid.
 | |
| Names | |
|---|---|
| IUPAC name
Hydrogen trithionate[1] | |
| Other names
trithionate(1−)[2] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
PubChem CID |
|
| Properties | |
| HO6S3−1[10] | |
| Molar mass | 193.18 g·mol−1 |
| Conjugate acid | Trithionic acid |
| Conjugate base | Trithionate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Team, EBI Web. "Chemical Entities of Biological Interest (ChEBI)". www.ebi.ac.uk. Retrieved 23 September 2018.
IUPAC Name hydrogen trithionate
- Team, EBI Web. "trithionate(1-) (CHEBI:33483)". www.ebi.ac.uk. Retrieved 23 September 2018.
ChEBI Name trithionate(1−)
- Team, EBI Web. "trithionate(1-) (CHEBI:33483)". www.ebi.ac.uk. Retrieved 23 September 2018.
SMILES [H]OS(=O)(=O)SS([O-])(=O)=O
- Team, EBI Web. "trithionate(1-) (CHEBI:33483)". www.ebi.ac.uk. Retrieved 23 September 2018.
InChI InChI=1S/H2O6S3/c1-8(2,3)7-9(4,5)6/h(H,1,2,3)(H,4,5,6)/p-1
- "Hydrogen trithionate". pubchem.ncbi.nlm.nih.gov. Retrieved 23 September 2018.
InChI InChI=1S/H2O6S3/c1-8(2,3)7-9(4,5)6/h(H,1,2,3)(H,4,5,6)/p-1
- Team, EBI Web. "trithionate(1-) (CHEBI:33483)". www.ebi.ac.uk. Retrieved 23 September 2018.
InChIKey KRURGYOKPVLRHQ-UHFFFAOYSA-M
- "Hydrogen trithionate". pubchem.ncbi.nlm.nih.gov. Retrieved 23 September 2018.
InChI Key: KRURGYOKPVLRHQ-UHFFFAOYSA-M
- "Hydrogen trithionate". pubchem.ncbi.nlm.nih.gov. Retrieved 23 September 2018.
InChI Key KRURGYOKPVLRHQ-UHFFFAOYSA-M
- Team, EBI Web. "trithionate(1-) (CHEBI:33483)". www.ebi.ac.uk. Retrieved 23 September 2018.
SMILES [H]OS(=O)(=O)SS([O-])(=O)=O
- Team, EBI Web. "trithionate(1-) (CHEBI:33483)". www.ebi.ac.uk. Retrieved 23 September 2018.
Net Charge -1
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.