Hydroelectric cell
Hydroelectric Cell (HEC) is a primary source of green energy device that generates electricity by dissociating/splitting water molecules on its surface.[1][2][3]
.jpg)
It was invented by Dr. R. K. Kotnala and Dr. Jyoti Shah in 2016 National Physical Laboratory of India, New Delhi.[4][5][6][7] Dissociation/Splitting of water molecules at oxygen deficient nonporous ferrite/oxide surface creates hydronium and hydroxide ions collected by Zn and Ag electrodes respectively to generate electricity. Hence combination of nonporous oxygen deficient magnesium ferrite/metal oxide along with Zn and Ag electrodes has been coined as Hydroelectric cell.[8][9] In fact, at room temperature dissociation/splitting of single water molecule requires 1.23 V potential, an external energy is required to split water molecule into its constituent ions that in general in conventional way is provided externally in the form of UV/catalysts, photons, thermal, biochemical etc.[10][11][12][13][14] On the other hand, Nano porous surface of ferrite/oxide material provides highly reactive surface for dissociation of water molecules in to its constituent ions at room temperature which is generally available in adsorbed state.[15][16]
The cell's Nano porous surface provides chemidissociated H+ and OH- ions at initial stage and subsequently followed by copious physidissociation due to high electric field developed inside nanopores resulting into continuous generation of large number of ions. Zn(Zinc) and Ag(Silver) electrodes attached on opposite surfaces of the ferrite/metal oxide pellet separate and collect opposite polarity ions result into flow of electric current in the cell. HEC is environment friendly, without producing any green house gases and its low cost raw materials are widely available as well as its disposal after its uses is non toxic. A two-inch diameter cell requires only few drops of water to develop a maximum voltage 0.98 V & a short circuit current of 120 mA. Results are highly comparable to solar cell and other portable electrical energy sources that too without use of any electrolyte/acid or alkali but requires only water. Hydroelectric cell different versions have been reported to produce green electricity using water droplets only.[17][18][19][20][21][22][23][24][25][26][27]
Working principle
Hydroelectric Cell (HEC) generates electricity by water splitting based on non-photo catalytic process and it is independent of the band gap of the material used in the cell. The cell works on the principle of water dissociation on nano-porous oxygen deficient ferrite/oxide pellet surface.The water molecules are initially chemi-dissociated at oxygen deficient nonporous surface into H+ and OH- ions and subsequently followed by continuous physi-dissociation by electrostatic field developed due to trapped H+ inside nano-pore on the pellet surface. Dissociated ions migrate towards Silver (Ag) inert cathode and Zinc (Zn) anode electrodes respectively and are collected via these electrodes for current generation in external circuits.In this process 0.98 V & 70 mA is generated in 4.5 sq.cm Hydroelectric cell. Zinc is consumed in this reaction and slowly converted into Zn(OH)2 and highly pure hydrogen gas is ejected. For any other electrolytic cell to work, generation of ions is essential, which is generally achieved by chemical reaction between electrolyte and electrode.Hydroelectric cell does not require any acid/alkali/electrolyte or light except water is required but only ferrite/metal oxide pellet surface acts as the continuous source of ions with water. Hydroelectric Cell working is a unique device based on the combination of material property, oxygen deficiency, Nano science and electro-chemistry.
Reaction mechanism
At Lithium substituted Magnesium Ferrite :
4H2O → 2H3O+ + 2OH−
at anode (Zn):
2OH− + Zn → Zn(OH)2 + 2e− (-0.76 V)
at cathode (Ag):
H3O+ + H3O+ + 2e− → H2↑ + 2H2O (+0.22 V)
Ecell = 0.22 -( -.76) = 0.98 V
Both by products of HEC are of commercial high value and the H2 gas as well as Zn(OH)2 Nano powder are biodegradable and environment friendly.
Design
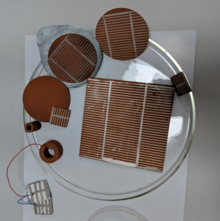
Fabrication of HEC includes obtaining of ferrite material by simple heat treatment by solid state reaction technique. A small (1 inch square) or large (2 inch diameter) pellet of ferrite/metal oxide material is coated with comb patterned silver electrodes on one surface and back surface is covered with Zinc electrode. Water is sprinkled on the surface of cell while current is collected via two electrodes in an external circuit.
Performance
HEC has been reported to produce maximum 0.125 A current at 0.98 V by 2 inch diameter circular pellet. All the raw materials that are being used in cell fabrication are cheap so it is cost effective choice for electricity production as compared to other resources. Hydroelectric Cell turns chemical energy into electrical energy. In fact it is the most efficient way to produce electricity by water.
Advantages
HEC generates green energy and the residues are non toxic and its low cost component raw materials. Others are:
- A HEC device is portable, compact and lightweight
- It requires minimal amount of water to produce energy with no usage of hazardous chemicals
- Unlike solar cell it can work during day or night and can run small scale devices like LED and fans continuously for couple of months at present which is improving further.
- The two byproducts of Hydroelectric cell, Hydrogen gas and Zn(OH)2 Nano-particles are environment friendly and can be utilized for other applications.
- It generates 99.9% pure H2 gas which can be used as a high quality fuel
- It is a reusable device
- No tedious manufacturing process is involved in making of HEC
- It is very clean and safe device is to be used for electricity production and is highly reliable with acceptable performance
Usage
- It can be used as a source for power generation at far/interior places like rural areas/farms, forests and mountains.
- Hydroelectric Cell can be utilized for domestic and residential applications in decentralized mode to generate electricity at small scale.
- After scaling up it can also be used to fulfill energy needs of automotive industry.
- At a large scale HEC finds its applications in consumer products specially in rural areas.
- One another unique application of HEC is to produce high purity Hydrogen gas.[28]
Youtube presence
Hydroelectric cell popularity among masses crossed over one million viewers in 18 months in YouTube besides millions of persons have watched TV News broadcast on HEC. Besides there have been lot of progress globally in past 3 years of Hydroelectric cell inception to be used in different applications.
References
- R..K. Kotnala; Jyoti Shah (6 June 2016). "Green hydroelectrical energy source based on water dissociation by nanoporous ferrite". International Journal of Energy Research. 40 (12): 1652–1661. doi:10.1002/er.3545.
- "Lithium-substituted magnesium ferrite material based hydroelectric cell and process for preparation thereof". Google Patents. Google Patents. Retrieved 27 May 2020.
- Kotnala, R. K.; Gupta, Rekha; Shukla, Abha; Jain, Shipra; Gaur, Anurag; Shah, Jyoti (2018-08-23). "Metal Oxide Based Hydroelectric Cell for Electricity Generation by Water Molecule Dissociation without Electrolyte/Acid". The Journal of Physical Chemistry C. American Chemical Society. 122 (33): 18841–18849. doi:10.1021/acs.jpcc.8b04999.
- "Indian scientists generate electricity from water sans using energy". aninews.in. Asian News International. Retrieved 31 May 2020.
- "Scientists urge for commercialization of Hydroelectricity for cheaper source of electrical energy". sciexaminer.com. sciexaminer. Retrieved 31 May 2020.
- "Indian Scientists Are Using Water To Generate Electricity". Scienceworldreport.com. Science World Report. Retrieved 31 May 2020.
- "Electricity-from-water scientist seeks commercialisation of invention". Economictimes.com. Economictimes. Retrieved 28 May 2020.
- Nzeogu, Uzo (1 January 2017). "Indian Scientists Generate 'Power' From Fresh Water". EnergyNews. EnergyNews. Retrieved 31 May 2020.
- Jain, Shipra; Shah, Jyoti; Dhakate, S. R.; Gupta, Govind; Sharma, C.; Kotnala, R. K. (20 February 2018). "Environment-Friendly Mesoporous Magnetite Nanoparticles-Based Hydroelectric Cell". The Journal of Physical Chemistry C. 122 (11): 5908–5916. doi:10.1021/acs.jpcc.7b12561.
- Hauch, Anne; Ebbesen, Sune Dalgaard; Jensen, Søren Højgaard; Mogensen, Mogens (20 June 2008). "Highly efficient high temperature electrolysis". The Journal of Material Chemistry. 18 (20): 2331–2340. doi:10.1039/B718822F.
- Geissler, Phillip L.; Dellago, Christoph; Chandler, David (21 April 1999). "Kinetic Pathways of Ion Pair Dissociation in Water". The Journal of Physical Chemistry B. 103 (18): 3706–3710. doi:10.1021/jp984837g.
- Rossmeisl, J.; Logadottir, A.; Nørskov, J.K. (7 December 2005). "Electrolysis of water on (oxidized) metal surfaces". Chemical Physics. 319 (1–3): 178–184. Bibcode:2005CP....319..178R. doi:10.1016/j.chemphys.2005.05.038.
- Del Valle, F.; Ishikawa, A.; Domen, K.; Villoria de la Mano, J.A.; Sánchez-Sánchez, M.C.; González, I.D.; Herreras, S.; Mota, N.; Rivas, M.E.; Álvarez Galván, M.C. (15 May 2009). "Influence of Zn concentration in the activity of Cd1−xZnxS solid solutions for water splitting under visible light". Catalysis Today. 142 (1–2): 51–56. doi:10.1016/j.cattod.2008.09.024.
- Kanan, M. W.; Nocera, D. G. (22 Aug 2008). "In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+". Science. 321 (5892): 1072–1075. Bibcode:2008Sci...321.1072K. doi:10.1126/science.1162018. PMID 18669820. S2CID 206514692.
- Parkinson, Gareth S.; Novotný, Zbyněk; Jacobson, Peter; Schmid, Michael; Diebold, Ulrike (8 July 2011). "Room Temperature Water Splitting at the Surface of Magnetite". Journal of the American Chemical Society. 133 (22): 12650–12655. doi:10.1021/ja203432e. PMID 21740022.
- Zografi, George (20 October 2008). "States of Water Associated with Solids". Drug Development and Industrial Pharmacy. 14 (14): 1905–1926. doi:10.3109/03639048809151997.
- Shah, Jyoti; Verma, K.C.; Agarwal, Ashish; Kotnala, R.K. (1 January 2020). "Novel application of multiferroic compound for green electricity generation fabricated as hydroelectric cell". Materials Chemistry and Physics. 239: 122068. doi:10.1016/j.matchemphys.2019.122068.
- Comprehensive Energy Systems (1 ed.). Netherland: ELSEVIER. 21 February 2018. pp. 223–230. ISBN 9780128095973. Retrieved 30 May 2020.
- Shah, Jyoti; Kumar Kotnala, Ravinder (September 2017). "Rapid green synthesis of ZnO nanoparticles using a hydroelectric cell without an electrolyte". Journal of Physics and Chemistry of Solids. 108: 15–20. Bibcode:2017JPCS..108...15S. doi:10.1016/j.jpcs.2017.04.007.
- Shah, Jyoti; Jain, Shipra; Shukla, Abha; Gupta, Rekha; Kotnala, Ravinder Kumar (28 December 2017). "A facile non-photocatalytic technique for hydrogen gas production by hydroelectric cell". International Journal of Hydrogen Energy. 42 (52): 30584–30590. doi:10.1016/j.ijhydene.2017.10.105.
- Jain, Shipra; Shah, Jyoti; Negi, Nainjeet Singh; Sharma, Chhemendra; Kotnala, Ravinder Kumar (6 June 2019). "Significance of Interface Barrier at Electrode of Hematite Hydroelectric Cell for Generating Eco-power by Water Splitting". International Journal of Energy Research. 43 (9): 4743–4755. doi:10.1002/er.4613.
- Solanki, V.; Krupanidhi, S. B.; Nanda, K. K. (5 September 2018). "Simultaneous water quality monitoring and degradation of hazardous organic pollutants". Review of Scientific Instruments. 89 (9): 096102. doi:10.1063/1.5041488. PMID 30278693.
- Kharbanda, Pranati; Madaan, Tushar; Sharma, Isha; Vashishtha, Shruti; Kumar, Parveen; Chauhan, Arti; Mittal, Sumit; Bangruwa, Jarnail S.; Verma, Vivek (24 January 2019). "Ferrites: magnetic materials as an alternate source of green electrical energy". Heliyon. 5 (1): 1151. doi:10.1016/j.heliyon.2019.e01151. PMC 6351576. PMID 30723829.
- Gobara, Heba M.; Nassar, Ibrahim M.; El Naggar, Ahmed M.A.; Eshaq, Gh. (1 January 2017). "Nanocrystalline spinel ferrite for an enriched production of hydrogen through a solar energy stimulated water splitting process". Energy. 118: 1234–1242. doi:10.1016/j.energy.2016.11.001.
- Solanki, Vanaraj; Krupanidhi, Saluru Baba; Nanda, Karuna Kar (25 November 2019). "Harvesting energy via stimuli‐free water/moisture dissociation by mesoporous SnO2–based hydroelectric cell and CuO as a pump for atmospheric moisture". International Journal of Energy Research. 44 (2): 1276–1283. doi:10.1002/er.4993.
- Chauhan, Shikha Singh; Gaur, Anurag; Kotnala, R. K. (March 2019). "Application of Hydroelectric Cell for LED Lamp". 2019 Innovations in Power and Advanced Computing Technologies (I-PACT). 2019 Innovations in Power and Advanced Computing Technologies (i-PACT). pp. 1–3. doi:10.1109/i-PACT44901.2019.8960035. ISBN 978-1-5386-8190-9. S2CID 210697518.
- Gaur, Anurag; Kumar, Anurag; Kumar, Purushottam; Agrawal, Rekha; Shah, Jyoti; Kotnala, Ravinder K. (12 May 2020). "Fabrication of a SnO2-Based Hydroelectric Cell for Green Energy Production". ACS Omega. 5 (18): 10240–10246. doi:10.1021/acsomega.9b03309. ISSN 2470-1343. PMC 7226856. PMID 32426580.
- Jain, Shipra; Shah, Jyoti; Negi, Nainjeet Singh; Sharma, Chhemendra; Kotnala, Ravinder Kumar (2019). "Significance of interface barrier at electrode of hematite hydroelectric cell for generating ecopower by water splitting". International Journal of Energy Research. 43 (9): 4743–4755. doi:10.1002/er.4613. ISSN 1099-114X.