Heptanal
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.[2]
| Names | |
|---|---|
| IUPAC name
Heptanal | |
| Other names
Heptanaldehyde Aldehyde C-7 Enanthaldehyde Heptyl aldehyde n-Heptanal | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.545 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H14O | |
| Molar mass | 114.18 |
| Appearance | Clear liquid |
| Density | 0.80902 at 30 °C |
| Melting point | −43.3 °C (−45.9 °F; 229.8 K) |
| Boiling point | 152.8 °C (307.0 °F; 425.9 K) |
| Slightly soluble | |
| -81.02·10−6 cm3/mol | |
| Related compounds | |
Related aldehydes |
Hexanal |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
The formation of heptanal in the fractional distillation of castor oil[3] was already described in 1878. The large-scale production is based on the pyrolytic cleavage of ricinoleic acid ester[4] (Arkema method) and on the hydroformylation of 1-hexene with rhodium 2-ethylhexanoate as a catalyst upon addition of some 2-ethylhexanoic acid (Oxea method):[2][5]
![]()
Heptanal naturally occurs in the essential oils of ylang-ylang (Cananga odorata), clary sage (Salvia sclarea), lemon (Citrus x limon), bitter orange (Citrus x aurantium), rose (Rosa) and hyacinth (Hyacinthus).[6]
Properties
Heptanal is a flammable, slightly volatile colorless liquid of pervasive fruity to oily-greasy odor,[7] which is miscible with alcohols[6] and practically insoluble in water.[8] Because of its sensitivity to oxidation, heptanal is filled under nitrogen and stabilized with 100 ppm hydroquinone.[9]
Heptanal forms flammable vapor-air mixtures. The compound has a flash point of 39.5 °C.[8] The explosion range is between 1.1% by volume as the lower explosion limit (LEL) and 5.2% by volume as the upper explosion limit.[8] Its ignition temperature is 205 °C.[8]
Uses
Heptanal can be used for the production of 1-heptanol via hydrogenation:

The oxidation of heptanal with oxygen in the presence of a rhodium catalysts leads at 50 °C to heptanoic acid in 95% yield.[10] Heptanal reacts with benzaldehyde in a Knoevenagel reaction under basic catalysis with high yield and selectivity (> 90%) to alpha-pentylcinnamaldehyde (also called jasmine aldehyde because of the typical jasmine odor),[11][2] which is mostly used in many fragrances as a cis/trans isomer mixture.[12]
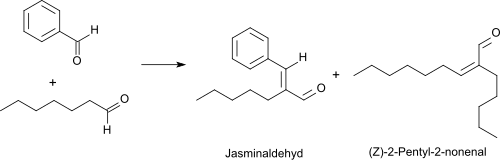
A by-product of the given reaction is the unpleasant rancid smelling (Z)-2-pentyl-2-nonenal.[13] When good reasons are given, heptanal can be converted into (Z)-2-pentyl-2-nonenal virtually quantitatively in the presence of aqueous boric acid upon azeotropic removal of water.[14]
![]()
Full hydrogenation provides the branched primary alcohol 2-pentylnonan-1-ol, also accessible from the Guerbet reaction from heptanol.[15]
References
- Merck Index, 11th Edition, 4578.
- Christian Kohlpaintner, Markus Schulte, Jürgen Falbe, Peter Lappe, Jürgen Weber. "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub2.CS1 maint: multiple names: authors list (link)
- F. Krafft, Distillation of castor oil, under educed pressure, Analyst, 3, 329a (1878).
- A. Chauvel, G. Lefebvre, Petrochemical Processes: Technical and Economic Characteristics, Band 2, S. 277, Editions Technip, Paris, 1989, ISBN 2-7108-0563-4.
- Deutsche Patentschrift DE 102007053385, Verfahren zur Herstellung von Aldehyden, Erfinder: A. Fischbach et al., Anmelder: Oxea Deutschland GmbH, veröffentlicht am 20. Mai 2009.
- G. A. Burdock, Fenaroli’s Handbook of Flavor Ingredients, Fifth Edition, 2005, CRC Press, Boca Raton, Fl., ISBN 0-8493-3034-3.
- "Richtwerte für gesättigte azyklische aliphatische C4- bis C11-Aldehyde in der Innenraumluft" (PDF). Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 52 (6): 650–659. 27 June 2009. doi:10.1007/s00103-009-0860-2.
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Acros Organics, Sicherheitsdatenblatt, Heptaldehyde, stabilized, überarb. am 19. November 2012.
- Deutsche Patentschrift DE 10010771, Verfahren zur Herstellung aliphatischer Carbonsäuren aus Aldehyden, Erfinder: H. Springer, P. Lappe, Anmelder: Celanese Chem Europe GmbH, veröffentlicht am 3. Mai 2001.
- Pérez-Sánchez, María; de María, Pablo Domínguez (2013). "Synthesis of natural fragrance jasminaldehyde using silica-immobilized piperazine as organocatalyst". Catalysis Science & Technology. 3 (10): 2732. doi:10.1039/C3CY00313B.
- Riechstofflexikon A, alpha-Amylzimtaldehyd, Letzte Änderung am 4. August 2000.
- J. M. Hornback, Organic Chemistry, 2nd edition, S. 886, Thomson Brooks/Cole, 2006, ISBN 0-534-49317-3.
- Offenhauer, Robert D.; Nelsen, Stephen F. (February 1968). "Aldehyde and ketone condensation reactions catalyzed by boric acid". The Journal of Organic Chemistry. 33 (2): 775–777. doi:10.1021/jo01266a059.
- G.H. Knothe: Lipid Chemistry, Guerbet Compounds Archived 2016-05-21 at the Wayback Machine, AOCS Lipid Library, 22. Dezember 2011.