Health Action International
Health Action International (HAI) is a non-profit organization based in The Netherlands. Established in 1981, HAI works to expand access to essential medicines through research, policy analysis and intervention projects. The organization focuses on snakebite envenoming, access to insulin and developing European policies on medicines. HAI is listed by the World Health Organization (WHO) as an official non-state actor.[1]
 | |
| Formation | 1981 |
|---|---|
| Type | Nonprofit NGO |
| Purpose | Access to medicines |
| Headquarters | Amsterdam, The Netherlands |
Region served | International |
Official language | English |
Executive Director | Dr Tim Reed |
Staff | 17 |
| Website | haiweb |
History
HAI was founded in Geneva in early 1981[2] following an International Baby Food Action Network[3] (IFBAN) conference and before a global conference on International Women and Health meeting to be held in Geneva.[4]:71[5] After limiting marketing of infant formulas to the third world, three groups came together to co-sponsor this meeting, the International Organization of Consumers Unions (IOCU),[6] the BUKO Pharma-Kampagne, an organization that watches over the marketing practices of German pharmaceutical companies and the United Nations Non-Governmental Liaison Service (NGLS),[7][5][8][4]:71 and brought together 50 anthropologists, physicians, pharmacists and organizers[3] from 27 countries to form an "international antibody"[3] against pharmaceutical marketing practices and used "innovative techniques to get their message to the delegates".[9]:139 It was similar to IFBAN but was organized to replace branded drugs with generics.[4]:72[10] The US Pharmaceutical Manufacturing Association said "we take this as a serious potential problem, both from a marketing threat here and now and for a WHO resolution in the future."[4]:72
HAI was immediately criticized by the pharmaceutical industry who implied a connection with Moscow[11] The German Pharmaceutical Manufacturing Association (BPI) published an article on the formation of HAI and connected it to a conference in November 1981 in Moscow of the World Federation of Trade Unions.[4]:72-74 Claus Roepnack, then CEO of a German pharmaceutical company, Hoechst AG, asked whether the activists of HAI meant to overthrow "existing social and economic systems in favor of authoritarian regimes".[4]:72[12] In 1987, the Thai newspaper The Nation made similar allegations.[4]:72
In 1982, HAI proposed a draft for “an international code on pharmaceuticals”[13] at the 35th World Health Assembly (WHA) meeting in an attempt to regulate the conduct of multinational drug companies,[4]:80-82 especially in developing countries.[10] The attempt was unsuccessful,[3] and the WHA declined to discuss the proposal.[10][8][14]
In 1984, HAI produced a video, Hard to Swallow, in collaboration with Oxfam about the experiences of Dianna Melrose of pharmaceutical sales rep practices in Peru.[4]:16 That same year, HAI lobbied the WHA delegates and published a number of publications to incorporate responsible drug use into the WHO's Essential Drugs Monitor. Not all of HAI's initiatives against the pharmaceutical industry were appreciated by the WHO.[4]:83-93
At one point, Asian operations were housed in Penang, Malaysia and a European operation was located in The Hague in the 90's but moved its headquarters to Amsterdam.[15]
A two-year project started in 1986 helped to reduce the use of Neomycin in antidiarrhetic products globally from 12% down to 7%.[4]:78
At the 41st World Health Assembly in 1987, HAI organised a large lobby of delegates to advocate for stronger controls on advertising by the pharmaceutical industry.[16] It advocated for an independent drug code similar to the US FDA but under the WHO which would replace the International Federation of Pharmaceutical Manufacturers & Associations drug marketing code.[15]
In 1988, HAI began discussions around issues with the Bamako Initiative.[4]:126-129 This initiative was a plan for UNICEF and other donors to supply drugs to Sub-Saharan African countries which would be sold a little above cost. The profits from these sales would be used to buy more drugs in a self-sustaining way. This work modified HAI's purpose to move towards health policy rather than just responsible medicine use.[4]:128
In 1989, the organization testified in front of the FDA arguing against the approval of Norplant, a hormone capsule implant developed by Wyeth-Ayerst Laboratories and designed as a long-acting contraceptive. It was approved and used by about a million women. HAI claimed that the implant had not been rigorously scientifically tested and warned of serious problems, among them its removal, infection at the implant site, and unknown long-term side effects.[17]
In response to 2001 WHA resolution, HAI together with the WHO a established a survey methodology to assess global drug prices and accessibility.[18][19][20][21][22]
Purpose
HAI works to increase access to essential medicines and encourage responsible medicine use.[8][2][7][23][10][24][3] Projects included neglected tropical diseases, access to insulin, sexual and reproductive health commodities, controlled medicines and transparency of medicine prices. According to Silverman et al. (1992), HAI and IOCU are the two organizations that have had the greatest impact on drug company activities in the third world.[15]
Snakebites
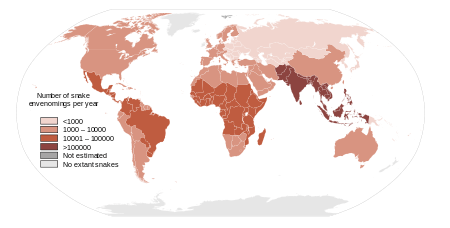
HAI works in Kenya, Uganda, Zambia and at the global level with WHO to gather snakebite incidence and treatments, including research and community education on first-aid and prevention.[25] HAI also works with local governments for policies to improved access to effective and safe antivenoms, and proper training for healthcare workers.[26][27] At the global level, HAI works with international partners such as the WHO Neglected Tropical Disease (NTD) Department and the Global Snakebite Initiative.[28][29][30] HAI helped obtain WHO Category A Neglected Tropical Disease (NTD) status for snakebites in 2018.[31][30]
Access to insulin
HAI produced a study of the global insulin market in 2018.[32] It then developed policies and tools to increase insulin access, and studied the cost of insulin production and estimated the number of people with type 2 diabetes.[33] HAI received a $3.5 million grant from The Leona M. and Harry B. Helmsley Charitable Trust in 2018 to study insulin access in low and middle income countries.[34]
European policy
HAI works to expand access to affordable medicines, promote medicine safety and enhance therapeutic value. HAI uses the TRIPS Agreement,[35][36] issuing policy recommendations on health technology assessment (HTA) and raising awareness on the impact of pharmaceutical marketing on prescribing behaviours.[37][38]
In 2011, HAI received a 218,000 Euro grant from the EU to look at strategies to improve access to medicines.[39]
References
- "Listing of HAI by the WHO as an official actor" (PDF). Retrieved 14 May 2019.
- Lee PR, Lurie P, Silverman MM, Lydecker M (1991). "Drug promotion and labeling in developing countries: an update". J Clin Epidemiol. 44 Suppl 2: 49S–55S. PMID 2045842.CS1 maint: multiple names: authors list (link)
- Greene, Jeremy (2011). "Making medicines essential: The emergent centrality of pharmaceuticals in global health". BioSocieties. 6 (1): 10–33. doi:10.1057/biosoc.2010.39.
- Chetley, Andrew (1990). A Healthy Business?: World Health and the Pharmaceutical Industry (1st ed.). pp. 71–73. ISBN 978-0862327354.
- Fazal, Anwar (1983). "The right pharmaceuticals at the right prices: Consumer perspectives". World Development. 11 (3): 265–269. doi:10.1016/0305-750X(83)90035-9. ISSN 0305-750X.
- "Encyclopaedia Britannica Entry on Consumers International". Encyclopaedia Britannica. Retrieved 17 May 2019.
- Reich, Michael R. (1987). "Essential drugs: economics and politics in international health". Health Policy. 8 (1): 39–57. doi:10.1016/0168-8510(87)90129-1.
- Laing R, Waning B, Gray A, Ford N, 't Hoen E (2003). "25 years of the WHO essential medicines lists: progress and challenges". Lancet. 361 (9370): 1723–9. doi:10.1016/S0140-6736(03)13375-2. PMID 12767751.CS1 maint: multiple names: authors list (link)
- Walt, Gil (1996). Health Policy: An Introduction to Process and Power. p. 139. ISBN 978-1856492645.
- Andia, Tatiana; Chorev, Nitsan (2017). "Making knowledge legitimate: transnational advocacy networks' campaigns against tobacco, infant formula and pharmaceuticals". Global Networks. 17 (2): 255–280. doi:10.1111/glob.12156. ISSN 1470-2266.
- Lofgren H (2004). "Pharmaceuticals and the consumer movement: the ambivalences of 'patient power'". Aust Health Rev. 28 (2): 228–37. doi:10.1071/AH040228. PMID 15527403.
- "Pharma Brief" (PDF). Bukopharma.de (in German) (4). 1986. Retrieved 15 May 2019.
- Patel, Surendra J. (1983). "Editor's introduction". World Development. 11 (3): 165–167. doi:10.1016/0305-750X(83)90021-9. ISSN 0305-750X.
- Halperin, Jerome A. (2016). "Multinational and International Regulation of Pharmaceuticals and US Policy". Drug Information Journal. 17 (3): 153–161. doi:10.1177/009286158301700304. ISSN 0092-8615.
- Silverman, Milton; Lydecker, Mia; Lee, Philip (1992). Bad Medicine: The Prescription Drug Industry in the Third World (1st ed.). Stanford University Press. pp. 185–188. ISBN 978-0804716697.
- "World Health Organization Essential Drugs Monitor" (PDF). apps.who.int. 1988.
- Duncan, Laura (Nov 1995). "Norplant: The Next Mass Tort". ABA Journal. 81.
- Van Puymbroeck RV (2010). "Basic survival needs and access to medicines--coming to grips with TRIPS: conversion + calculation". J Law Med Ethics. 38 (3): 520–49. doi:10.1111/j.1748-720X.2010.00510.x. PMID 20880239.
- Fang Y, Wagner AK, Yang S, Jiang M, Zhang F, Ross-Degnan D (2013). "Access to affordable medicines after health reform: evidence from two cross-sectional surveys in Shaanxi Province, western China". Lancet Glob Health. 1 (4): e227-37. doi:10.1016/S2214-109X(13)70072-X. PMID 25104348.CS1 maint: multiple names: authors list (link)
- Anson A, Ramay B, de Esparza AR, Bero L (2012). "Availability, prices and affordability of the World Health Organization's essential medicines for children in Guatemala". Global Health. 8: 22. doi:10.1186/1744-8603-8-22. PMC 3503802. PMID 22747646.CS1 maint: multiple names: authors list (link)
- Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R (2009). "Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis". Lancet. 373 (9659): 240–9. doi:10.1016/S0140-6736(08)61762-6. PMID 19042012.CS1 maint: multiple names: authors list (link)
- Sharma A, Bhandari PM, Neupane D, Kaplan WA, Mishra SR (2018). "Challenges constraining insulin access in Nepal-a country with no local insulin production". Int Health. 10 (3): 182–190. doi:10.1093/inthealth/ihy012. PMID 29617832.CS1 maint: multiple names: authors list (link)
- Walt G (1993). "WHO under stress: implications for health policy". Health Policy. 24 (2): 125–44. doi:10.1016/0168-8510(93)90030-S. PMID 10126754.
- "CAMPAIGNING FOR STRONGER DRUG POLICIES". The Lancet. 322 (8364): 1435–1436. 1983. doi:10.1016/S0140-6736(83)90975-3. ISSN 0140-6736.
- Ratcliffe, Rebecca (May 24, 2018). "Mambas, medicine and one girl's race to survive Kenya's biting problem". The Guardian. The Guardian. Retrieved 14 May 2019.
- Eveleens, Ilona (August 21, 2018). "Slangenbeten zijn een vergeten ziekte ten zuiden van de Sahara" (in Dutch). de Verdieping Trouw. de Verdieping Trouw. Retrieved 14 May 2019.
- "La morsure de serpent, un fléau oublié qui empoisonne toujours l'Afrique" (in French). Le Monde. Le Monde. March 19, 2019. Retrieved 14 May 2019.
- Chakraborty, Ajanta (February 23, 2019). "New WHO strategy aims to halve the global impact of snakebite". The Times of India. The Times of India. Retrieved 14 May 2019.
- MCFARLING, USHA LEE (12 June 2017). "Snakebite finally makes a WHO list of top global health priorities". STAT. Retrieved 14 May 2019.
- Harrison RA, Casewell NR, Ainsworth SA, Lalloo DG (2019). "The time is now: a call for action to translate recent momentum on tackling tropical snakebite into sustained benefit for victims". Trans R Soc Trop Med Hyg. doi:10.1093/trstmh/try134. PMID 30668842.CS1 maint: multiple names: authors list (link)
- "Striking Back: Snakebites Gain Global Attention". plos.org. May 8, 2018. Retrieved 14 May 2019.
- Boseley, Sarah (20 Nov 2018). "Insulin shortage could affect 40 million people with type 2 diabetes". The Guardian. The Guardian. Retrieved 14 May 2019.
- Lay, Kat (25 September 2018). "NHS paying too much for insulin". The Times. Retrieved 14 May 2019.
- "$3.5 million grant from The Leona M. and Harry B. Helmsley Charitable Trust". The Leona M. and Harry B. Helmsley Charitable Trust. Retrieved 17 May 2019.
- Lee, Kelley; Buse, Kent; Fustukian, Suzanne (2002). Health Policy in a Globalising World (1st ed.). Cambridge: Cambridge University Press. p. 91. ISBN 978-0521009430. Retrieved 15 May 2019.
- Fletcher, Elaine (16 May 2018). "TRIPS Flexibilities in High Demand". Health Policy Watch. Retrieved 14 May 2019.
- Ed Silverman, Ed (2018-03-20). "Pharma payments to docs in Europe are often inadequately reported or hard to find". STAT. Retrieved 14 May 2019.
- Francesca Bruce, Francesca (1 February 2018). "Mandatory Joint Clinical Assessments For EU HTAs May Be 'Counterproductive'". Pink Sheet Pharma Intelligence. Retrieved 14 May 2019.
- "EU Health Programme: 2011 Call for Proposals, Abstracts of Actions selected for funding" (PDF). European Commission. Retrieved 17 May 2019.