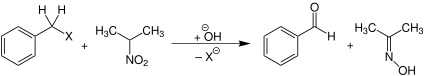Hass–Bender oxidation
In organic chemistry, the Hass–Bender oxidation (also called the Hass–Bender carbonyl synthesis[1]) is an organic oxidation reaction that converts benzyl halides into benzaldehydes using the sodium salt of 2-nitropropane as the oxidant.[2] This name reaction is named for Henry B. Hass and Myron L. Bender, who first reported it in 1949.[3]
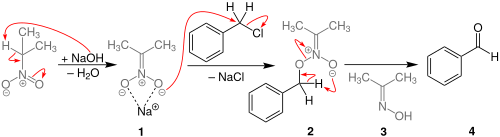
The reaction process begins with the deprotonation of 2-nitropropane at the α carbon to form a resonance-stabilized anion. This compound then initiates an SN2 reaction to displace the benzyl halide. Unlike in the nitroaldol reaction, where the deprotonated carbon of the nitroalkyl group is the nucleophilic atom, it is instead an oxygen of the nitro itself that attacks the benzylic carbon.[4] The O-benzyl structure then undergoes a pericyclic reaction to produce a benzaldehyde, with dimethyloxime as a byproduct.
Although originally developed for benzyl compounds, the reaction also works for allyl halides, giving the respective α,β-enones and enals.[5]
References
- Hassner, Alfred; Namboothiri, Irishi (2012). "Hass–Bender carbonyl synthesis". Organic Syntheses Based on Name Reactions: A Practical Guide to 750 Transformations. Elsevier. pp. 203–204. ISBN 978-0-08-096630-4.
- Wang, Zerong (2009). "Hass–Bender oxidation". Comprehensive Organic Name Reactions and Reagents. Wiley. pp. 1335–1337. doi:10.1002/9780470638859.conrr297. ISBN 978-0-471-70450-8.
- Hass, Henry B.; Bender, Myron L. (1949). "The Reaction of Benzyl Halides with the Sodium Salt of 2-Nitropropane. A General Synthesis of Substituted Benzaldehydes". J. Am. Chem. Soc. 71 (5): 1767–1769. doi:10.1021/ja01173a066.
- Bersohn, Malcolm (1961). "C versus O Alkylation in the Case of a Stable Cation". J. Am. Chem. Soc. 83 (9): 2136–2138. doi:10.1021/ja01470a022.
- Montavon, M.; Lindlar, H.; Marbet, R.; Rüegg, R.; Ryser, G.; Saucy, G.; Zeller, P.; Isler, O. (1957). "Synthesen in der Carotinoid‐Reihe. 11. Mitteilung. α,β‐Ungesättigte Carbonylverbindungen aus Allylhalogeniden mittels Nitroparaffinen". Helv. Chim. Acta. 40 (5): 1250–1256. doi:10.1002/hlca.19570400516.