Haplozoon
Haplozoon (/hæploʊ’zoʊən/) are unicellular endo-parasites, primarily infecting maldanid polychaetes. They belong to Dinoflagellata but differ from typical dinoflagellates. Most dinoflagellates are free-living and possess two flagella. Instead, Haplozoon belong to a 5% minority of parasitic dinoflagellates that are not free-living. Additionally, the Haplozoon trophont stage is particularly unique due to an apparent lack of flagella. The presence of flagella or remnant structures is the subject of ongoing research.
| Haplozoon | |
|---|---|
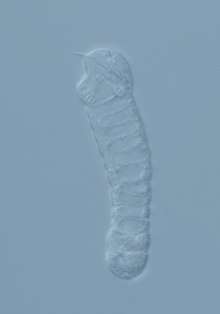 | |
| Light micrograph of Haplozoon axiothellae in its trophont stage | |
| Scientific classification | |
| Domain: | |
| (unranked): | |
| (unranked): | |
| Phylum: | |
| Subphylum: | Dinozoa |
| Infraphylum: | |
| Class: | |
| Order: | Blastodiniales |
| Family: | Haplozoonidae |
| Genus: | Haplozoon |
At first glance, Haplozoon also do not appear unicellular – in fact they were originally classified as a possible transitional stage between protists and multicellular organisms.[1] They have more recently been classified as compartmentalized syncytia – single cells with multiple nuclei that have been subdivided by internal membranes.[2] Their life cycle is also largely unknown; while there is a well-observed adult trophont stage, understanding of other life stages is speculative at best.
There is a single published case of Haplozoon infecting an appendicularian.[3] Otherwise, they are almost exclusively documented as infecting maldanids, and the extent to which they are an appendicularian parasite has not been investigated.
Etymology
Haplozoon derives from two Greek words: haploos meaning “single, or simple” and zoion meaning “animal”. When they were first discovered, they were initially placed within Mesozoa, a group of highly reduced worm-like parasitic invertebrates. Therefore, the genus name Haplozoon means “simple animal”.
History of Knowledge
The type species Haplozoon armatum was discovered by Russian zoologist Valentin Dogiel in 1906. Dogiel initially described Haplozoon as belonging to the Mesozoa, and established a new mesozoan class, the Catenata.[4]
A series of early protistologists continued to fine-tune the taxonomical position of Haplozoon for a number of years.[1] The following year, the French biologist Édouard Chatton indicated similarities between Haplozoon and Blastodinium in 1907. Later, in 1911, the German naturalist Franz Poche created a new protozoan class – the Haplozooidea. And again in 1920, Chatton created a new family (Haplozoonidae) for the genus, of the order Gymnodinida (modern syn. Gymnodiniales). By the 1970s Haplozoon had been moved from the Gymnodiniales to the order of Blastodiniales.[5]
Our modern understanding of Haplozoon is limited. There were a series of purported species discoveries in the early 1900s, however these publications lack the modern techniques that would be mandatory today (e.g. transmission electron microscopy, scanning electron microscope, molecular sequencing, etc.). In some cases, basic information such as the host species, or location of discovery, have not been recorded. The unfortunate result is that many purported species of Haplozoon are phantom species. Phantom species are those that have not been seen since their initial discovery, often due to an inadequate original description.
Only 5 species of Haplozoon have been sufficiently described by modern standards: Haplozoon axiothellae,[2][5][6] Haplozoon praxillellae,[7] Haplozoon ezoense[8], Haplozoon gracile[9], and Haplozoon pugnus[9].
Habitat and ecology
Haplozoon are obligate parasites. They are almost exclusively found in the gut of maldanid marine worms, with one study documenting a Haplozoon parasite infecting an appendicularian.[3] Each trophont has a stylet, which it uses to pierce the gut lining of the host worm. It is unclear if the stylet is used only for anchoring the parasite, or if it is involved in feeding as well. If Haplozoon do use their stylet to feed, this would be referred to as myzocytosis, and commonly referred to as “cellular vampirism”. This is an established feeding method among other alveolate parasites.[10][11]
Another possible feeding strategy is that the parasite absorbs nutrients that are released as the host worm digests food. This feeding strategy is referred to as pinocytosis, a form of endocytosis where nutrients suspended in the fluid if the host's gut are absorbed through the cell membrane of the parasite. The exterior of Haplozoon cells are covered by barbs that present as fine hair-like structures that might function in surface mediated nutrition similar to the microtriches of cestoda.[2]
With few exceptions, most descriptions of Haplozoon are from European coastlines. Haplozoon clymenellae is from the Atlantic coast of North America,[1] while H. axiothellae, and H. praxillellae have been found on the Pacific coast of Washington, US and British Columbia, Canada.[2][5][7] H. ezoense, H. gracile, and H. pugnus were discovered on the coast of Japan.[8][9] There are currently no recorded observations from the tropics or the southern hemisphere, and consequently little is known about its biogeographical distribution.
Description of the organism
Morphology
What makes Haplozoon unique among dinoflagellates is their functional multicellularity. H. axiothellae cells are composed of a series of compartments, which give them the appearance of multicellularity. At minimum all Haplozoon species consist of a single row of compartments, with mature cells of some species containing two or more rows of compartments at the posterior of their cell. These compartments are specialized for different functions, such as host attachment, feeding and reproduction. The degree of compartmentalization varies by species.[8]
The Haplozoon trophont (i.e. the stage that feeds on the host) has 3 types of compartments: (1) a trophocyte used to attach to the host, (2) repeating gonocytes that compose the majority of the cell length, and (3) sporocytes which develop from mature gonocytes. The anterior-most compartment of the cell is the trophocyte, larger than the gonocytes that are aligned immediately posterior to it. The rear most compartments are referred to as sporocytes, and depending on the species of Haplozoon, sporocytes may remain in single file (e.g. H. lineare), form double rows (e.g. H. axiothellae) or even multiple rows (e.g. H. clymenellae). All three compartment types appear granular under light microscopy, due to starch granules in their cytoplasm.[8]
Originally, Haplozoon were described as having 2-26 “cells”.[1] They vary in size by species, but the most well-studied species (H. axiothellae) is described as being 40-175 μm in length, and 15-40 μm wide.
Haplozoon lack visible flagella, where traditional dinoflagellates have two – one transverse and one longitudinal. H. axiothellae possesses a longitudinal row of ventral pores along the cell surface, a possible vestigial feature from lost flagella.[2] The exterior of Haplozoon cells are covered in amphiesmal projections (syn. thecal barbs).
The trophocyte attaches to the gut lining of the host by means of its stylet and a suction cup apparatus. Some species of Haplozoon are known to possess a single stylet (H. ezoense), where others are known to have multiple (reserve) stylets within the trophocyte (H. axiothellae, H. praxillellae). Some species also appear to have “arms” that extend from the trophocyte.[2][8]
Early transmission electron microscopy (TEM) briefly describes 3 layers of the cellular membrane. They describe a single continuous outer layer equivalent to a cell membrane, with two subsequent layers below of compressed “thecal vesicles”.[6] Today these “thecal vesicles” are known as alveoli, or amphiesmal vesicles.[12] The divisions between gonocytes are described as consisting of outer continuous membranes and flattened vesicles but pressed so closely together they are impossible to distinguish.[6] The divisions between compartments are now understood to be created by interlocking amphiesmal vesicles rather than cellular membranes.[8] This solidified the description of Haplozoon as compartmentalized syncytia, rather than a multicellular organism.
All compartments contain their own nuclei, with some gonocytes being binucleate and some sporocytes being quadrinucleate.[1] The nuclei of Haplozoon have thick chromosomes,[8] typical of Dinokaryota. Mitochondria are mainly located below the amphiesmal vesicles towards the outside of the cell and possess tubular cristae.[6][8]
TEM sections have revealed multiple triple membrane-bound organelles within the gonocytes.[8] They are roughly spherical and range in size from 200 nm – 750 nm. They appear like relic non-photosynthetic plastids, which have been found among other members of Myzozoa (apicomplexans as well as dinoflagellates). This is consistent with our understanding that some lineages of dinoflagellates have experienced plastid loss as they evolved.[13] This discovery in Haplozoon only provides ultrastructure evidence for these remnant plastids; no research has been published to assess plastid genes and any associated metabolic pathways.
Life cycle
Very little is confirmed about the Haplozoon life cycle; the only life-stage that is known is the trophont. Given similarities with other dinoflagellate parasites, it is hypothesized that the known trophont stage is the “adult”.
It is presumed that the sporocytes are what allow the organism to reproduce, but this has not been shown definitively. It is believed that the sporocytes produced at the posterior end of the trophont become motile dinospores, which are then released through the anus of the host. Once in the marine environment, the dinospores can be ingested by a new host, and subsequently develop into an adult trophont and attach themselves to the gut of their new host.
Dinospores were described in Haplozoon dogieli.[1] Sporocytes were observed detaching from adult trophonts and developing into small flagellated dinospores.[1] They were 12 μm in length and similar to the dinospores of Oodinium, Apodinium, and Blastodinium. These dinospores were also observed quickly encysting if disturbed. Subsequent studies have been unable to reproduce the dinospore or cyst life stages.[5][9] Thus, no image data capturing these stages currently exists. While the existence of dinospores is likely, they have not been observed since 1924.
Phylogeny
Modern dinoflagellate phylogenies recognize 8 orders: Syndiniales, Gymnodiniales, Noctilucales, Suessiales, Peridiniales, Gonyaulacales, Prorocentrales, and Dinophysiales.[14] But this has not always been the case – early in the study of dinoflagellates, other orders were used such as Blastodiniales.[15] Originally, Blastodiniales was a group for all parasitic dinoflagellates.
Through the years, Blastodiniales has fallen out of favour and use. Subsequent phylogenetic analyses have confirmed that Blastodiniales is a polyphyletic group, whose existence is not supported by modern molecular data.[16] There is a history of former members of Blastodiniales being moved to new orders based on molecular data.[17][18]
As of June 2020, molecular data exists for five Haplozoon species: H. axiothellae, H. praxillellae, H. ezoense, H. gracile, and H. pugnus.[7][8][13][9] Beyond being dinoflagellates, 18S phylogenetic analyses have failed to resolve the position of Haplozoon. Therefore, Haplozoon remain in the polyphyletic order Blastodiniales, awaiting conclusive molecular data that confirms its true phylogenetic position.
The following species are known to exist:[19]
- H. armatum, Dogiel, 1906 (host: Travisia forbesi)
- H. lineare, Dogiel, 1907 (host: Clymene lumbricalis)
- H. delicatulum, Dogiel, 1910 (Host: maldanid, gen sp?)
- H. ariciae, Dogiel, 1910 (host: Aricia norvegica)
- H. macrostylum, Dogiel, 1910 (host: maldanid, gen sp?)
- H. obscurum, Dogiel, 1910 (host: Terebellides strömii)
- H. clymenellae, Calkins, 1915 (host: Clymenella torquata)[1]
- H. clymenidis, Dogiel & Mikelsson, 1923 (host: Euclymene palermitana)
- H. tuberculatum, Dogiel & Mikelsson 1923 (host: maldanid gen sp?)
- H. villosum, Dogiel & Mikelsson 1923 (host: Polyphysia crassa, formerly Eumenia crassa)
- H. dogielii, Shumway, 1924 (host: Leiochone clypeata)
- H. inerme, Cachon 1964 (host: Appendicularia sicula)
- H. axiothellae, Siebert, 1973 (host: Axiothellae rubrocincta)[2][5][6]
- H. praxillellae, Rueckert and Leander, 2008 (host: Praxillella pacifica)[7]
- H. ezoense, Wakeman, 2018 (host: Praxillella pacifica)[8]
- H. gracile, Yamamoto, 2020 (host: cf. Petaloclymene sp.)[9]
- H. pugnus, Yamamoto, 2020 (host: Nicomache personata, Nicomache sp.)[9]
References
- Shumway, W. (1924). The genus Haplozoon, Dogiel. Observations on the life history and systematic position. The Journal of Parasitology, 11(2), 59-74.
- Leander, B., Saldarriaga, J., & Keeling, P. (2002). Surface morphology of the marine parasite Haplozoon axiothellae Siebert (Dinoflagellata). European Journal of Protistology, 38(3), 287–297. doi: 10.1078/0932-4739-00882
- Cachon J. (1964). Contribution a l’étude des Péridiniens parasites. Cytologie. Cycles évolutifs. Ann. Sci. Nat. Zool. 12: 1-158.
- Dogiel, V. (1906). Haplozoon armatum n. gen. nova sp., der Vertreter einer neuen Mesozoa-Gruppe. Zool. Anz., (30), 895-899.
- Siebert, A. (1973). A description of Haplozoon axiothellae n. sp., an endosymbiont of the polychaete Axiothellae rubrocincta. Journal of Phycology, 9(2), 185-190. doi: 10.1111/j.1529-8817.1973.tb04077.x
- Siebert, A., & West, J. (1974). The fine structure of the parasitic dinoflagellate Haplozoon axiothellae. Protoplasma, 81(1), 17-35. doi: 10.1007/BF02055771
- Rueckert, S., & Leander, B. (2008). Morphology and molecular phylogeny of Haplozoon praxillellae n. sp. (Dinoflagellata): a novel intestinal parasite of the maldanid polychaete Praxillella. European Journal of Protistology, 44(4), 299-307. doi: 10.1016/j.ejop.2008.04.004
- Wakeman, K., Yamaguchi, A., & Horiguchi, T. (2018). Molecular phylogeny and morphology of Haplozoon ezoense n. sp. (Dinophyceae): A parasitic dinoflagellate with ultrastructural evidence of remnant non-photosynthetic plastids. Protist, 169(3), 333-350. doi: 10.1016/j.protis.2018.04.008
- Yamamoto, Mana, et al. "Molecular phylogeny and ultrastructure of two novel parasitic dinoflagellates, Haplozoon gracile sp. nov. and H. pugnus sp. nov." Phycologia (2020): 1-15.
- Gómez, F., Moreira, D., & López-García, P. (2009). Life cycle and molecular phylogeny of the dinoflagellates Chytriodinium and Dissodinium, ectoparasites of copepod eggs. European Journal of Protistology, 45(4), 260-270. doi: 10.1016/j.ejop.2009.05.004
- Cavalier-Smith, T., & Chao, E. E. (2004). Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). European Journal of Protistology, 40(3), 185-212. doi: 10.1016/j.ejop.2004.01.002
- Cavalier-Smith, T. (1991). Cell diversification in heterotrophic flagellates. The Biology of Free-living Heterotrophic Flagellates. 113-131.
- Saldarriaga, J., Taylor, F., Keeling, P., & Cavalier-Smith, T. (2001). Dinoflagellate nuclear SSU rRNA phylogeny suggests multiple plastid losses and replacements. Journal of Molecular Evolution, 53(3), 204–213. doi: 10.1007/s002390010210
- Janouškovec, J., Gavelis, G. S., Burki, F., Dinh, D., Bachvaroff, T. R., Gornik, S. G., Bright, K. J., Imanian, B., Strom, S. L., Delwiche, C. F. and Waller, R. F. (2017). Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics. Proceedings of the National Academy of Sciences, 114(2), E171-E180. doi: 10.1073/pnas.1614842114
- Fensome, R. A, Taylor F. J. R., Norris G., Sarjeant W. A. S., Wharton D. I. and Williams G. L. (1993). A Classification of Living and Fossil Dinoflagellates. Micropaleontology, special publication, 7, 1-351.
- Saldarriaga, J., Cavalier-Smith, T., Menden-Deuer, S., & Keeling, P. (2004). Molecular data and the evolutionary history of dinoflagellates. European Journal of Protistology, 40(1), 85-111. doi: 10.1016/j.ejop.2003.11.003
- Skovgaard, A., Massana, R., & Saiz, E. (2007). Parasitic species of the genus Blastodinium (Blastodiniphyceae) are Peridinioid Dinoflagellates 1. Journal of Phycology, 43(3), 553-560. doi: 10.1111/j.1529-8817.2007.00338.x
- Gómez, F., & Skovgaard, A. (2015). Molecular phylogeny of the parasitic dinoflagellate Chytriodinium within the Gymnodinium clade (Gymnodiniales, Dinophyceae). Journal of Eukaryotic Microbiology, 62(3), 422-425. doi: 10.1111/jeu.12180
- Gómez, F. (2012). "A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata)." Cicimar Oceánides, 27(1), 65-140.