HEPACAM
Gene HEPACAM*, named based on its original site of identification - hepatocytes and the nature of its protein product - a cell adhesion molecule (CAM), was first discovered and characterised in human liver and reported by Shali Shen (MD, PhD) in 2005.[5] The gene encodes a protein of 416 amino acids, designated as hepaCAM**, which is a new member of the immunoglobulin superfamily of cell adhesion molecules (IgSF CAM). The main biological functions of hepaCAM include a) modulating cell-matrix adhesion and migration, and b) inhibiting cancer cell growth.[5]
| HEPACAM | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | HEPACAM, GlialCAM, MLC2A, MLC2B, hepatic and glial cell adhesion molecule, HEPACAM1, HEPN1 | ||||||||||||||||||||||||
| External IDs | OMIM: 611642 MGI: 1920177 HomoloGene: 17652 GeneCards: HEPACAM | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
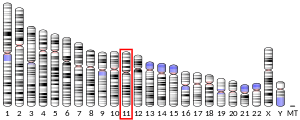
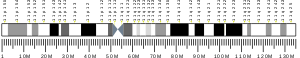
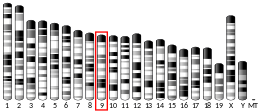
| Location (UCSC) | Chr 11: 124.92 – 124.94 Mb | Chr 9: 37.37 – 37.39 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
(Note: *HEPACAM, gene name; **hepaCAM, protein name)
Discovery
Through differential screening of gene expression, over 200 genes were found to be either up- or down-regulated in a hepatocellular carcinoma patient. These genes were subsequently evaluated against a panel of human HCC specimens, leading to the identification of a novel gene HEPN1.[6] Based on the sequence of HEPN1, the new gene HEPACAM was then isolated and characterised.[7]
Characteristics and functions
Structurally, hepaCAM is a glycoprotein containing an extracellular domain with 2 Ig-like loops, a transmembrane region and a cytoplasmic domain.[7] Matched to chromosome 11q24, gene HEPACAM is ubiquitously expressed in normal human tissues, with particularly high expression levels in the central nervous system (CNS), and is frequently suppressed in a variety of tumour types.[8] Functionally, hepaCAM is involved in cell-extracellular matrix interactions and growth control of cancer cells,[7] and is able to induce differentiation of glioblastoma cells.[9] In cell signaling, hepaCAM directly interacts with F-actin[10] and calveolin 1,[11] and is capable of inducing senescence-like growth arrest via a p53/p21-dependent pathway.[8] Moreover, hepaCAM is proteolystically cleaved near the transmemberane region.[12] These findings indicate that the new Ig-like cell adhesion molecule hepaCAM is also a tumour suppressor.[13]
Other names
About HEPACAM 2
Metastatic canine mammary carcinoma and their metastases are characterized by decreased HEPACAM2 but unchanged HEPACAM2 expression levels when compared to normal glands.[15]
References
- GRCh38: Ensembl release 89: ENSG00000165478 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000046240 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Chung Moh M, Hoon Lee L, Shen S (June 2005). "Cloning and characterization of hepaCAM, a novel Ig-like cell adhesion molecule suppressed in human hepatocellular carcinoma". Journal of Hepatology. 42 (6): 833–41. doi:10.1016/j.jhep.2005.01.025. PMID 15885354.
- Moh MC, Lee LH, Yang X, Shen S (October 2003). "HEPN1, a novel gene that is frequently down-regulated in hepatocellular carcinoma, suppresses cell growth and induces apoptosis in HepG2 cells". Journal of Hepatology. 39 (4): 580–6. doi:10.1016/S0168-8278(03)00359-3. PMID 12971969.
- Moh MC, Zhang C, Luo C, Lee LH, Shen S (July 2005). "Structural and functional analyses of a novel ig-like cell adhesion molecule, hepaCAM, in the human breast carcinoma MCF7 cells". The Journal of Biological Chemistry. 280 (29): 27366–74. doi:10.1074/jbc.M500852200. PMID 15917256.
- Moh MC, Zhang T, Lee LH, Shen S (December 2008). "Expression of hepaCAM is downregulated in cancers and induces senescence-like growth arrest via a p53/p21-dependent pathway in human breast cancer cells". Carcinogenesis. 29 (12): 2298–305. doi:10.1093/carcin/bgn226. PMID 18845560.
- Lee LH, Moh MC, Zhang T, Shen S (August 2009). "The immunoglobulin-like cell adhesion molecule hepaCAM induces differentiation of human glioblastoma U373-MG cells". Journal of Cellular Biochemistry. 107 (6): 1129–38. doi:10.1002/jcb.22215. PMID 19507233.
- Moh MC, Tian Q, Zhang T, Lee LH, Shen S (May 2009). "The immunoglobulin-like cell adhesion molecule hepaCAM modulates cell adhesion and motility through direct interaction with the actin cytoskeleton". Journal of Cellular Physiology. 219 (2): 382–91. doi:10.1002/jcp.21685. PMID 19142852.
- Moh MC, Lee LH, Zhang T, Shen S (January 2009). "Interaction of the immunoglobulin-like cell adhesion molecule hepaCAM with caveolin-1". Biochemical and Biophysical Research Communications. 378 (4): 755–60. doi:10.1016/j.bbrc.2008.11.119. PMID 19059381.
- Zhang T, Moh MC, Lee LH, Shen S (July 2010). "The immunoglobulin-like cell adhesion molecule hepaCAM is cleaved in the human breast carcinoma MCF7 cells". International Journal of Oncology. 37 (1): 155–65. doi:10.3892/ijo_00000663. PMID 20514407.
- Moh MC, Shen S (2009). "The roles of cell adhesion molecules in tumor suppression and cell migration: a new paradox". Cell Adhesion & Migration. 3 (4): 334–6. doi:10.4161/cam.3.4.9246. PMC 2802741. PMID 19949308.
- Favre-Kontula L, Rolland A, Bernasconi L, et al. (April 2008). "GlialCAM, an immunoglobulin-like cell adhesion molecule is expressed in glial cells of the central nervous system". Glia. 56 (6): 633–45. doi:10.1002/glia.20640. PMID 18293412.
- Klopfleisch R, Klose P, da Costa A, Brunnberg L, Gruber AD (2010). "HEPACAM1 and 2 are differentially regulated in canine mammary adenomas and carcinomas and its lymph node metastases". BMC Veterinary Research. 6: 15. doi:10.1186/1746-6148-6-15. PMC 2842258. PMID 20226097.