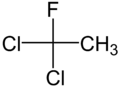
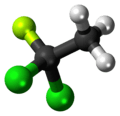
1,1-Dichloro-1-fluoroethane
1,1-Dichloro-1-fluoroethane is a haloalkane with the formula C
2H
3Cl
2F. It is one of the three isomers of dichlorofluoroethane. It belongs to the hydrochlorofluorocarbon family (HCFC).
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1-Dichloro-1-fluoroethane | |||
| Other names
Dichlorofluoroethane; R-141b; HCFC-141b | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.100.575 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 9274 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H3Cl2F | |||
| Molar mass | 116.94 g·mol−1 | ||
| Appearance | Colorless liquid, ethereal odor | ||
| Density | 1.25 g/cm3 at 20 °C[1] | ||
| Melting point | −103.5 °C (−154.3 °F; 169.7 K)[1] | ||
| Boiling point | 32 °C (90 °F; 305 K)[1] | ||
| 4 g/L (20 °C)[1] | |||
| Hazards | |||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H412, H420 | ||
| P273, P501, P502 | |||
| 532 °C (990 °F; 805 K)[1] | |||
| Explosive limits | 5.6–17.7% vol.[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
5 g/kg (rat, oral) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Use
1,1-Dichloro-1-fluoroethane is mainly used as a refrigerant under the names R-141b or HCFC-141b.
Physico-chemical properties
It is a non-flammable, colourless liquid in atmospheric conditions. The compound is very volatile and its smell has been described as "usually ethereal".
gollark: The screens are excellently sized for it though.
gollark: Like showing ads to bricks.
gollark: Yes.
gollark: Humans typically have greater depth than bricks. But I don't have a brick on hand or a ruler to verify.
gollark: You're paying for things *with shifts in your attitudes and perceptions*.
References
- Record of 1,1-Dichloro-1-fluoroethane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 8 February 2009.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.