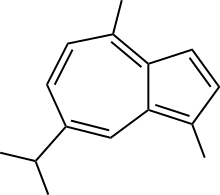
Guaiazulene
Guaiazulene, also azulon or 1,4-dimethyl-7-isopropylazulene, is a dark blue crystalline hydrocarbon. A structural isomer of azulene, guaiazulene is a bicyclic sesquiterpene that is a constituent of some essential oils, mainly oil of guaiac and chamomile oil, which also serve as its commercial sources. Various soft corals also contain guaiazulene as a principal pigment.[1] Its low melting point makes guaiazulene difficult to handle, in contrast to the crystalline nature of the parent azulene. The electronic structure (and colors) of guaiazulene and azulene are very similar.

 | |
| Names | |
|---|---|
| IUPAC name
1,4-Dimethyl-7-isopropylazulene | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.002 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H18 | |
| Molar mass | 198.309 g·mol−1 |
| Density | 0.976 g/cm3 |
| Melting point | 31 to 33 °C (88 to 91 °F; 304 to 306 K) |
| Boiling point | 153 °C (307 °F; 426 K) (7 mm Hg) |
| Pharmacology | |
| S01XA01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
Guaiazulene is an U.S. FDA-approved cosmetic color additive. It – or its 3-sulfonate – is a component of some skin care products together with other skin soothing compounds such as allantoin.[3]
Guaiazulene has applications as a volatile dye with a known evaporation rate to indicate end of use of various products (such as insecticide strips).
References
- Bowden, BF; Coll, JC; Tapiolas, DM (1983). "Studies of Australian soft corals. XXX. A novel trisnorsesquiterpene from a Cespitulariaspecies and the isolation of guaiazulene from a small blue Alcyoniumspecies". Australian Journal of Chemistry. 36: 211. doi:10.1071/CH9830211.
- Harmon AD, Weisgraber KH, Weiss U.; Weisgraber; Weiss (1979). "Preformed azulene pigments of Lactarius indigo (Schw.) Fries (Russulaceae, Basidiomycetes)". Cellular and Molecular Life Sciences. 36 (1): 54–56. doi:10.1007/BF02003967. ISSN 1420-682X.CS1 maint: multiple names: authors list (link)
- Guarrera, M; Turbino, L; Rebora, A (2001). "The anti-inflammatory activity of azulene". Journal of the European Academy of Dermatology and Venereology. 15 (5): 486. doi:10.1046/j.1468-3083.2001.00340.x. PMID 11763400.