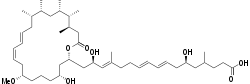
Gladiolin
Gladiolin is a polyketide natural product produced by Burkholderia gladioli BCC0238 which is isolated from sputum of cystic fibrosis patients. It was found to be a novel macrolide antibiotic which presented an activity against Mycobacterium tuberculosis.[1] Gladiolin is structurally much more stable than its analogue etnangien[2] as an efficient myxobacterial RNA polymerase inhibitor due to the lack of highly labile hexaene moiety in gladiolin.[1] The good activity and high stability of gladiolin offers it the potential for further development as an antibiotic against antibiotic-resistant M. tuberculosis.
 | |
| Identifiers | |
|---|---|
| Properties | |
| C44H74O11 | |
| Molar mass | 779.065 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potential uses
Because of the structure similarity between gladiolin and etnangien, gladiolin was proved to inhibit RNA polymerase,[1] which is a validated drug target in M. tuberculosis including isoniazid- and rifampicin-resistant M. tuberculosis clinical isolates.[3] Gladiolin also exhibits low mammalian cytotoxicity and high stability.
History
Burkholderia is a prolific producer of many antimicrobial compounds, for example, thailanstatin,[4] spliceostatin,[5] phytotoxin, rhizoxin,[6] and others. Gladiolin was also discovered in another Burkholderia species, Burkholderia gladioli BCC0238, which was first isolated in 1996 from the sputum of a child with cystic fibrosis. The discovery and biosynthesis of gladiolin was first reported in May 2017 by University of Warwick and Cardiff University.[1] They also claimed that gladiolin presents promising activity against M. tuberculosis.
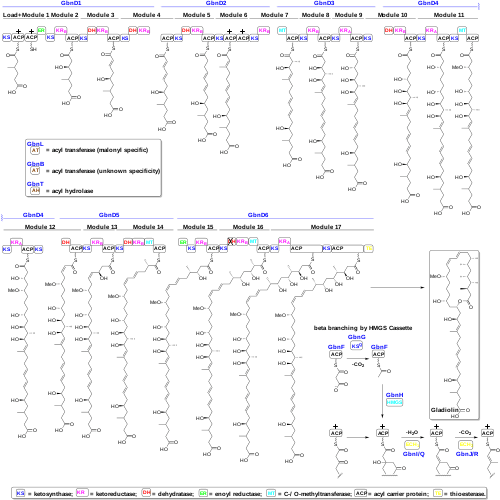
Biosynthesis
Gladiolin is assembled by trans-acyltransferase polyketide synthase (PKS). The gladiolin PKS contains 20 KS domains, 17 of which are predicted to catalyze chain elongation, the rest of them act as transacylases. The initiation step was proposed to be transacylation of the succinyl moiety of succinyl-CoA onto the active site Cys residue in the N-terminal ketosynthase domain of GbnD1 and was supported by phylogenetic analyses.[1]
References
- Song, Lijiang; Jenner, Matthew; Masschelein, Joleen; Jones, Cerith; Bull, Matthew J.; Harris, Simon R.; Hartkoorn, Ruben C.; Vocat, Anthony; Romero-Canelon, Isolda; Coupland, Paul; Webster, Gordon; Dunn, Matthew; Weiser, Rebecca; Paisey, Christopher; Cole, Stewart T.; Parkhill, Julian; Mahenthiralingam, Eshwar; Challis, Gregory L. (5 June 2017). "Discovery and Biosynthesis of Gladiolin: A Antibiotic with Promising Activity against". Journal of the American Chemical Society. 139 (23): 7974–7981. doi:10.1021/jacs.7b03382. PMID 28528545.
- Menche, Dirk; Arikan, Fatih; Perlova, Olena; Horstmann, Nicole; Ahlbrecht, Wiebke; Wenzel, Silke C.; Jansen, Rolf; Irschik, Herbert; Müller, Rolf (29 October 2008). "Stereochemical Determination and Complex Biosynthetic Assembly of Etnangien, a Highly Potent RNA Polymerase Inhibitor from the Myxobacterium Sorangium cellulosum". Journal of the American Chemical Society. 130 (43): 14234–14243. doi:10.1021/ja804194c.
- Eker, Barbara; Ortmann, Johannes; Migliori, Giovanni B.; Sotgiu, Giovanni; Muetterlein, Ralf; Centis, Rosella; Hoffmann, Harald; Kirsten, Detlef; Schaberg, Tom; Ruesch-Gerdes, Sabine; Lange, Christoph (November 2008). "Multidrug- and Extensively Drug-Resistant Tuberculosis, Germany". Emerging Infectious Diseases. 14 (11): 1700–1706. doi:10.3201/eid1411.080729. PMC 2630755. PMID 18976552.
- Liu, Xiangyang; Biswas, Sreya; Berg, Michael G.; Antapli, Christopher M.; Xie, Feng; Wang, Qi; Tang, Man-Cheng; Tang, Gong-Li; Zhang, Lixin; Dreyfuss, Gideon; Cheng, Yi-Qiang (21 March 2013). "Genomics-Guided Discovery of Thailanstatins A, B, and C As Pre-mRNA Splicing Inhibitors and Antiproliferative Agents from MSMB43". Journal of Natural Products. 76 (4): 685–693. doi:10.1021/np300913h. PMC 3696399. PMID 23517093.
- He, Haiyin; Ratnayake, Anokha S.; Janso, Jeffrey E.; He, Min; Yang, Hui Y.; Loganzo, Frank; Shor, Boris; O’Donnell, Christopher J.; Koehn, Frank E. (6 August 2014). "Cytotoxic Spliceostatins from sp. and Their Semisynthetic Analogues". Journal of Natural Products. 77 (8): 1864–1870. doi:10.1021/np500342m. PMID 25098528.
- Partida-Martinez, L. P.; Groth, I.; Schmitt, I.; Richter, W.; Roth, M.; Hertweck, C. (1 November 2007). "Burkholderia rhizoxinica sp. nov. and Burkholderia endofungorum sp. nov., bacterial endosymbionts of the plant-pathogenic fungus Rhizopus microsporus". International Journal of Systematic and Evolutionary Microbiology. 57 (11): 2583–2590. doi:10.1099/ijs.0.64660-0. PMID 17978222.