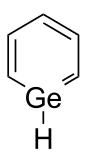
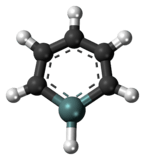
Germabenzene
Germabenzene (C5H6Ge) is the parent representative of a group of chemical compounds containing in their molecular structure a benzene ring with a carbon atom replaced by a germanium atom. Germabenzene itself has been studied theoretically,[1] and synthesized with a bulky 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl or Tbt group.[2] Also, stable naphthalene derivatives do exist in the laboratory such as the 2-germanaphthalene-containing substance represented below.[3] The germanium to carbon bond in this compound is shielded from potential reactants by a Tbt group. This compound is aromatic just as the other carbon group representatives silabenzene and stannabenzene.
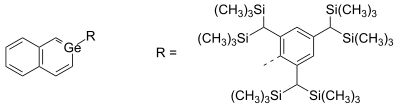 A stable 2-germanaphthalene derivative
A stable 2-germanaphthalene derivative
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Germine | |||
| Other names
Germanabenzene; Germin; Germanin | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H6Ge | |||
| Molar mass | 138.733 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
See also
- 6-membered aromatic rings with one carbon replaced by another group: borabenzene, benzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, pyrylium salt
References
- Ebrahimi, A. A.; Ghiasi, R.; Foroutan-Nejad, C. (2010). "Topological Characteristics of the Ring Critical Points and the Aromaticity of Groups IIIa to VIa Hetero-Benzenes". Journal of Molecular Structure: THEOCHEM. 941 (1–3): 47–52. doi:10.1016/j.theochem.2009.10.038.
- Nakata, Norio; Takeda, Nobuhiro; Tokitoh, Norihiro (2002-06-01). "Synthesis and Properties of the First Stable Germabenzene". Journal of the American Chemical Society. 124 (24): 6914–6920. doi:10.1021/ja0262941. ISSN 0002-7863.
- Nakata, N.; Takeda, N.; Tokitoh, N. (2001). "Synthesis and Structure of a Kinetically Stabilized 2-Germanaphthalene: The First Stable Neutral Germaaromatic Compound". Organometallics. 20 (26): 5507–5509. doi:10.1021/om010881y.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.