Germacrene
Germacrenes are a class of volatile organic hydrocarbons, specifically, sesquiterpenes. Germacrenes are typically produced in a number of plant species for their antimicrobial and insecticidal properties, though they also play a role as insect pheromones. Two prominent molecules are germacrene A and germacrene D.
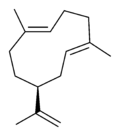 | |
| Names | |
|---|---|
| IUPAC name
(1E,5E,8S)-1,5-dimethyl-8-(prop-1-en-2-yl)cyclodeca-1,5-diene | |
| Other names
(−)-Germacrene A
Germacra-3,9,11-triene, (E,E)- 1,5-cyclodecadiene, 1,5-dimethyl-8-(1-methylethenyl)-, (1E,5E,8S)- 1,5-cyclodecadiene, 1,5-dimethyl-8-(1-methylethenyl)-, (S-(E,E))- | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 6500908 (A) 1864177 (D) | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.35 g/mol |
| Density | 0.793 g/mL |
| Boiling point | 236.4 °C (457.5 °F; 509.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
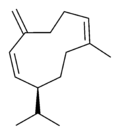 | |
| Names | |
|---|---|
| IUPAC name
(S,1Z,6Z)-8-isopropyl-1-methyl-5-methylenecyclodeca-1,6-diene | |
| Other names
1-methyl-5-methylene-8-(1-methylethyl)-1,6-cyclodecadiene
1,6-cyclodecadiene, 1-methyl-5-methylene-8-(1-methylethyl)- | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.35 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
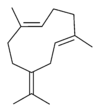
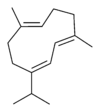
Structures
Germacrene has five isomers.
 |
 |
 |
 |
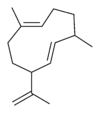 |
| A | B | C | D | E |
Natural occurrences
The essential oils of red deadnettle (Lamium purpureum)[1] and hedgenettles (genus Stachys)[2] are characterized by their high contents of germacrene D, as is Clausena anisata.
gollark: You'd also need your UPID, but you can just call an observation drone to scan your DNA and neuron imprint.
gollark: Well, you'd need your phone to access GTech™.
gollark: Yes, GTech™ is the only relevant one.
gollark: GTech™'s IIDB allows lookup by UPID (universal PotatOS ID) which would work.
gollark: Internal GTech™ APIs. You don't have an API key.
References
- Flamini G, Cioni PL, Morelli I (2005). "Composition of the essential oils and in vivo emission of volatiles of four Lamium species from Italy: L. purpureum, L. hybridum, L. bifidum and L. amplexicaule". Food Chemistry. 91 (1): 63–68. doi:10.1016/j.foodchem.2004.05.047.
- Katayoun Morteza‐Semnani, Mohammad Akbarzadeh, Shayesteh Changizi (2005-11-01). "Essential oils composition of Stachys byzantina, S. inflata, S. lavandulifolia and S. laxa from Iran". Flavor and Fragrance Journal. doi:10.1002/ffj.1594.CS1 maint: uses authors parameter (link)
General
- Adio AM (2009). "Germacrenes A–E and related compounds: thermal, photochemical and acid induced transannular cyclizations". Tetrahedron. 65 (8): 1533–1552. doi:10.1016/j.tet.2008.11.050.
Germacrene A
- Deguerry F, Pastore L, Wu S, Clark A, Chappell J, Schalk M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch Biochem Biophys. 2006 Oct 15;454(2):123-36.
- Omura H, Honda K, Feeny P. From terpenoids to aliphatic acids: further evidence for late-instar switch in osmeterial defense as a characteristic trait of swallowtail butterflies in the tribe papilionini. J Chem Ecol. 2006 Sep;32(9):1999-2012.
- Forcat S, Allemann RK. Stabilisation of transition states prior to and following eudesmane cation in aristolochene synthase. Org Biomol Chem. 2006 Jul 7;4(13):2563-7.
- Bertea CM, Voster A, Verstappen FW, Maffei M, Beekwilder J, Bouwmeester HJ. Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library. Arch Biochem Biophys. 2006 Apr 15;448(1-2):3-12.
- Lou Y, Baldwin IT. Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 2006 Mar;140(3):1126-36.
- Chang YJ, Jin J, Nam HY, Kim SU. Point mutation of (+)-germacrene A synthase from Ixeris dentata. Biotechnol Lett. 2005 Mar;27(5):285-8.
Germacrene D
- Rivero-Cruz B, Rivero-Cruz I, Rodriguez JM, Cerda-Garcia-Rojas CM, Mata R. Qualitative and quantitative analysis of the active components of the essential oil from Brickellia veronicaefolia by nuclear magnetic resonance spectroscopy. J Nat Prod. 2006 Aug;69(8):1172-6.
- Yang FQ, Li SP, Chen Y, Lao SC, Wang YT, Dong TT, Tsim KW. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2005 Sep 15;39(3-4):552-8.
- Umlauf D, Zapp J, Becker H, Adam KP. Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae). Phytochemistry. 2004 Sep;65(17):2463-70.
- Agnihotri VK, Thappa RK, Meena B, Kapahi BK, Saxena RK, Qazi GN, Agarwal SG. Essential oil composition of aerial parts of Angelica glauca growing wild in North-West Himalaya (India). Phytochemistry. 2004 Aug;65(16):2411-3.
- Raal A, Paaver U, Arak E, Orav A. Content and composition of the essential oil of Thymus serpyllum L. growing wild in Estonia. Medicina (Kaunas). 2004;40(8):795-800.
- He X, Cane DE. Mechanism and stereochemistry of the germacradienol/germacrene D synthase of Streptomyces coelicolor A3(2). J Am Chem Soc. 2004 Mar 10;126(9):2678-9.
- Arimura, G-I., Huber, DPW, Bohlmann, J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1. The Plant Journal 2004 37: 603-616.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.