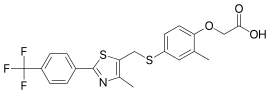
GW501516
GW501516 (also known as GW-501,516, GW1516, GSK-516, Cardarine, and on the black market as Endurobol[1]) is a PPARδ receptor agonist that was invented in a collaboration between Ligand Pharmaceuticals and GlaxoSmithKline in the 1990s, was entered into clinical development as a drug candidate for metabolic diseases and cardiovascular diseases, and was abandoned in 2007 because animal testing showed that the drug caused cancer to develop rapidly in several organs.[2]
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H18F3NO3S2 |
| Molar mass | 453.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
In 2007, research was published showing that high doses of GW501516 given to mice dramatically improved their physical performance; the work was widely discussed in popular media, and led to a black market for the drug candidate and to its abuse by athletes as a doping agent. The World Anti-Doping Agency (WADA) developed a test for GW501516 and other related chemicals and added them to the prohibited list in 2009; it has issued additional warnings to athletes that GW501516 is not safe.
History
GW501516 was initially discovered during a research collaboration between GSK and Ligand Pharmaceuticals that began in 1992.[3] The discovery of the compound was published in a 2001 issue of PNAS.[4] Oliver et al. reported that they used "combinatorial chemistry and structure-based drug design" to develop it.[5] One of the authors was the son of Leo Sternbach who discovered benzodiazepines in the 1960s.[6]
R & D Focus Drug News reported that GSK began phase I trials of the compound for the treatment of hyperlipidemia in 2000[7] followed by phase I/II in 2002.[8] In 2003, Ligand Pharmaceuticals earned a $1 million payment as a result of GSK continuing phase I development.[9]
By 2007, GW501516 had completed two phase II clinical studies and other studies relating to obesity, diabetes, dyslipidemia and cardiovascular disease,[10][11] but GSK abandoned further development of the drug in 2007 for reasons which were not disclosed at the time.[12] It later emerged that the drug was discontinued because animal testing showed that the drug caused cancer to develop rapidly in several organs, at dosages of 3 mg/kg/day in both mice and rats.[2][13][14]
Ronald M. Evans's laboratory purchased a sample of GW501516 and gave mice a much higher dose than had been used in GSK's experiments; they found that the compound dramatically increased the physical performance of the mice. The work was published in 2007 in Cell and was widely reported in the popular press including The New York Times and The Wall Street Journal.[15]
Performance-enhancing drug
Concerns were raised prior to the 2008 Beijing Olympics that GW501516 could be used by athletes as an ergogenic performance-enhancing drug that was not currently controlled by regulations or detected by standard tests. One of the main researchers from the study on enhanced endurance consequently developed a urine test to detect the drug, and made it available to the International Olympic Committee. The World Anti-Doping Agency (WADA) developed a test for GW501516 and other related PPARδ modulators,[16] and added such drugs to the prohibited list in 2009.[17]
GW501516 has been promoted on bodybuilding and athletics websites[18] and by 2011 had already been available for some time on the black market.[1][19] In 2011, it was reported to cost $1,000 for 10 g.[15] In 2012, WADA recategorised GW501516 from a gene doping compound to a "hormone and metabolic modulator".[20]
In 2013, WADA took the rare step of warning potential users of the compound of the possible health risks, stating that "clinical approval has not, and will not be given for this substance"; the New Scientist attributed the warning to the risks of the drug causing cancer.[18][21]
A number of athletes have tested positive for GW501516. At the Vuelta Ciclista a Costa Rica in December 2012, four Costa Rican riders tested positive for GW501516. Three of them received two-year suspensions, while the fourth received 12 years as it was his second doping violation.[22][23][24] In April 2013, Russian cyclist Valery Kaykov was suspended by cycling's governing body UCI after having tested positive for GW501516. Kaykov's team RusVelo dismissed him immediately[25] and in May 2013, Venezuelan Miguel Ubeto was provisionally suspended by the Lampre team.[26] In February 2014, Russian race walker Elena Lashmanova tested positive for GW501516.[27][28] In April 2019, heavyweight boxer Jarrell Miller tested positive for GW501516 which caused his challenge for Anthony Joshua's World Heavyweight titles to be cancelled.
Mode of action
GW501516 is a selective agonist (activator) of the PPARδ receptor.[29] It displays high affinity (Ki = 1 nM) and potency (EC50 = 1 nM) for PPARδ with > 1,000 fold selectivity over PPARα and PPARγ.[5]
In rats, binding of GW501516 to PPARδ recruits the coactivator PGC-1α. The PPARδ/coactivator complex in turn upregulates the expression of proteins involved in energy expenditure.[30] Furthermore, in rats treated with GW501516, increased fatty acid metabolism in skeletal muscle and protection against diet-induced obesity and type II diabetes was observed. In obese rhesus monkeys, GW501516 increased high-density lipoprotein (HDL) and lowered very-low-density lipoprotein (VLDL).[30]
See also
References
- "Anti-doping agency warns cheats on the health risks of Endurobol". The Conversation. 2013-03-22.
- Sahebkar A, Chew GT, Watts GF (2014). "New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease". Expert Opin Pharmacother. 15 (4): 493–503. doi:10.1517/14656566.2014.876992. PMID 24428677.
Despite these promising early results, the further investigation and development of GW501516 was discontinued after observations in animal studies of its association with the rapid induction of cancers in several organs (liver, stomach, tongue, skin, bladder, ovaries, womb and testes
- "GW501516 GlaxoSmithKline, Ligand milestone payment". R & D Focus Drug News. 28 June 2004.
- Wolf G (November 2003). "The function of the nuclear receptor peroxisome proliferator-activated receptor delta in energy homeostasis". Nutr. Rev. 61 (11): 387–90. doi:10.1301/nr.2003.nov.387-390. PMID 14677574. S2CID 12362203.
- Oliver WR, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM (April 2001). "A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport". Proc. Natl. Acad. Sci. U.S.A. 98 (9): 5306–11. Bibcode:2001PNAS...98.5306O. doi:10.1073/pnas.091021198. PMC 33205. PMID 11309497.
- Julia Flynn (11 February 2004). "Father and Son: In Two Generations, Drug Research Sees a Big Shift". The Wall Street Journal.
- "GW501516 Glaxo Wellcome phase change I, UK". R & D Focus Drug News. 20 November 2000.
- "GW501516 GlaxoSmithKline phase change II, UK". R & D Focus Drug News. 25 February 2002.
- "Ligand Pharmaceuticals Incorporated Earns $1 Million Milestone Payment as GlaxoSmithKline Advances Development of 501516". Reuters Significant Developments. 5 June 2003.
- Barish GD, Narkar VA, Evans RM (March 2006). "PPAR delta: a dagger in the heart of the metabolic syndrome". J. Clin. Invest. 116 (3): 590–7. doi:10.1172/JCI27955. PMC 1386117. PMID 16511591.
- Uwe Dressel; Tamara L. Allen; Jyotsna B. Pippal; Paul R. Rohde; Patrick Lau & George E. O. Musc (2003). "The Peroxisome Proliferator-Activated Receptor β/δ Agonist, GW501516, Regulates the Expression of Genes Involved in Lipid Catabolism and Energy Uncoupling in Skeletal Muscle Cells". Molecular Endocrinology. 17 (12): 2477–93. doi:10.1210/me.2003-0151. PMID 14525954.
- Billin AN (October 2008). "PPAR-beta/delta agonists for Type 2 diabetes and dyslipidemia: an adopted orphan still looking for a home". Expert Opin Investig Drugs. 17 (10): 1465–71. doi:10.1517/13543784.17.10.1465. PMID 18808307.
- Geiger LE, Dunsford WS, Lewis DJ, Brennan C, Liu KC, Newsholme SJ (2009). PS 895 - Rat carcinogenicity study with GW501516, a PPAR delta agonist (PDF). 48th Annual Meeting of the Society of Toxicology. Baltimore: Society of Toxicology. p. 105. Archived from the original (PDF) on 2015-05-04.
- Newsholme SJ, Dunsford WS, Brodie T, Brennan C, Brown M, Geiger LE (2009). PS 896 - Mouse carcinogenicity study with GW501516, a PPAR delta agonist (PDF). 48th Annual Meeting of the Society of Toxicology. Baltimore: Society of Toxicology. p. 105. Archived from the original (PDF) on 2015-05-04.
- Michael Bezar (2011-11-01). "Faster. Higher. Squeakier". Outside magazine. Retrieved 2013-04-02.
- Laurance J, Rajan A (2008-08-01). "Warning to Beijing Olympics over pills that mimic exercise". Health News, Health & Wellbeing. The Independent. Retrieved 2008-08-01.
- WADA 2009 Prohibited List Archived February 3, 2009, at the Wayback Machine
- "Anti-doping agency warns athletes of black market drug". New Scientist. 2013-03-26.
- Thevis M, Geyer H, Thomas A, Schänzer W (May 2011). "Trafficking of drug candidates relevant for sports drug testing: detection of non-approved therapeutics categorized as anabolic and gene doping agents in products distributed via the Internet". Drug Test Anal. 3 (5): 331–6. doi:10.1002/dta.283. PMID 21538997.
- Sanchis-Gomar F, Lippi G (March 2012). "Telmisartan as metabolic modulator: a new perspective in sports doping?". J Strength Cond Res. 26 (3): 608–10. doi:10.1519/JSC.0b013e31824301b6. PMID 22130396.
- "WADA issues alert on GW501516". World Anti-Doping Agency. 2013-03-21. Archived from the original on June 2, 2013.
- Shane Stokes: GW501516 positives confirmed, three of four riders are from same BCR Pizza Hut team, velonation.com, 15 April 2013
- Shane Stokes: Four riders each handed two year bans for use of GW501516, velonation.com 30 July 2013
- List of sanctions Archived July 15, 2014, at the Wayback Machine, uci.ch
- "European champion Valery Kaykov sacked for failing drug test". BBC. 2013-04-11. Retrieved 2013-04-11.
- "Miguel Ubeto Aponte provisionally suspended". UCI. 2013-05-13. Archived from the original on 2013-06-28. Retrieved 2013-05-15.
- Sanctioned athletes list – 26 June 2014
- Associated Press: Doping probe launched into Russian walkers, espn.com, 11 July 2014
- Pelton P (April 2006). "GW501516 GlaxoSmithKline/Ligand". Curr Opin Investig Drugs. 7 (4): 360–70. PMID 16625823.
- Sprecher DL (December 2007). "Lipids, lipoproteins, and peroxisome proliferator activated receptor-delta". Am. J. Cardiol. 100 (11 A): n20–4. doi:10.1016/j.amjcard.2007.08.009. PMID 18047848.