Frank-Kamenetskii theory
In combustion, Frank-Kamenetskii theory explains the thermal explosion of a homogeneous mixture of reactants, kept inside a closed vessel with constant temperature walls. It is named after a Russian scientist David A. Frank-Kamenetskii, who along with Nikolay Semenov developed the theory in the 1930s.[1][2][3][4]
Problem description[5][6][7][8][9]
Consider a vessel maintained at a constant temperature , containing a homogeneous reacting mixture. Let the characteristic size of the vessel be . Since the mixture is homogeneous, the density is constant. During the initial period of ignition, the consumption of reactant concentration is negligible (see and below), thus the explosion is governed only by the energy equation. Assuming a one-step global reaction , where is the amount of heat released per unit mass of fuel consumed, and reaction rate governed by Arrhenius law, the energy equation becomes
where
- is the temperature of the mixture
- is the specific heat at constant volume
- is the thermal conductivity
- is the pre-exponential factor with dimension of one over time
- is the initial fuel mass fraction
- is the activation energy
- is the universal gas constant
Non-dimensionalization
The non-dimensional activation energy and the heat-release parameter are
The characteristic heat conduction time across the vessel is , the characteristic fuel consumption time is and the characteristic explosion/ignition time is . Note should be made that in combustion process, typically so that . Therefore, , i.e., the fuel is consumed at much longer times when compared with ignition time, the fuel consumption is essentially negligible to study ignition/explosion. That is the reason the fuel concentration is assumed to same as the initial fuel concentration . The non-dimensional scales are
where is the Damköhler number and is the spatial coordinate with origin at the center, for planar slab, for cylindrical vessel and for spherical vessel. With this scale, the equation becomes
Since , the exponential term can be linearized , hence
Semenov theory
Before Frank-Kamenetskii, his doctoral advisor Nikolay Semyonov (or Semenov) proposed a thermal explosion theory with a simple model i.e., he assumed a linear function for heat conduction process instead of Laplacian operator. Semenov's equation reads as
For , the system explodes since the exponential term dominates. For , the system goes to a steady state, the system doesn't explode. In particular, Semenov found the critical Damköhler number, which is called as Frank-Kamenetskii parameter (where ) as a critical point where the system changes from steady state to explosive state. For , the solution is
At time , the system explodes. This time is also referred to as the adiabatic induction period since heat conduction is negligible here.
Frank-Kamenetskii steady-state theory[10][11]
The only parameter which characterizes the explosion is the Damköhler number . When is very high, conduction time is longer than the chemical reaction time and the system explodes with high temperature since there is not enough time for conduction to remove the heat. On the other hand, when is very low, heat conduction time is much faster than the chemical reaction time, such that all the heat produced by the chemical reaction is immediately conducted to the wall, thus there is no explosion, it goes to an almost steady state, Amable Liñán coined this mode as slowly reacting mode. At a critical Damköhler number the system goes from slowly reacting mode to explosive mode. Therefore, , the system is in steady state. Instead of solving the full problem to find this , Frank-Kamenetskii solved the steady state problem for various Damköhler number until the critical value, beyond which no steady solution exists. So the problem to be solved is
with boundary conditions
the second condition is due to the symmetry of the vessel. The above equation is special case of Liouville–Bratu–Gelfand equation in mathematics.
Planar vessel
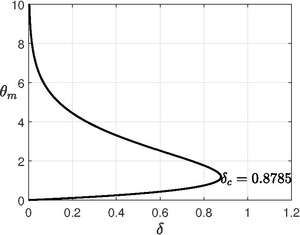
For planar vessel, there is an exact solution. Here , then
If the transformations and , where is the maximum temperature which occurs at due to symmetry, are introduced
Integrating once and using the second boundary condition, the equation becomes
and integrating again
The above equation is the exact solution, but maximum temperature is unknown, but we have not used the boundary condition of the wall yet. Thus using the wall boundary condition at , the maximum temperature is obtained from an implicit expression,
Critical is obtained by finding the maximum point of the equation (look figure), i.e., at .
So the critical Frank-Kamentskii parameter is . The system has no steady state( or explodes) for and for , the system goes to a steady state with very slow reaction.
Cylindrical vessel
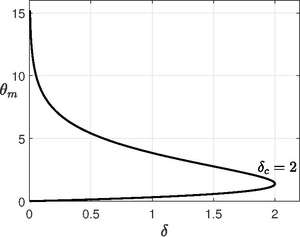
For cylindrical vessel, there is an exact solution. Though Frank-Kamentskii used numerical integration assuming there is no explicit solution, Paul L. Chambré provided an exact solution in 1952.[12] H. Lemke also solved provided a solution in a somewhat different form in 1913[13]. Here , then
If the transformations and are introduced
The general solution is . But from the symmetry condition at the centre. Writing back in original variable, the equation reads,
But the original equation multiplied by is
Now subtracting the last two equation from one another leads to
This equation is easy to solve because it involves only the derivatives, so letting transforms the equation
This is a Bernoulli differential equation of order , a type of Riccati equation. The solution is
Integrating once again, we have where . We have used already one boundary condition, there is one more boundary condition left, but with two constants . It turns out and are related to each other, which is obtained by substituting the above solution into the starting equation we arrive at . Therefore, the solution is
Now if we use the other boundary condition , we get an equation for as . The maximum value of for which solution is possible is when , so the critical Frank-Kamentskii parameter is . The system has no steady state( or explodes) for and for , the system goes to a steady state with very slow reaction. The maximum temperature occurs at
For each value of , we have two values of since is multi-valued. The maximum critical temperature is .
Spherical vessel
For spherical vessel, there is no known explicit solution, so Frank-Kamenetskii used numerical methods to find the critical value. Here , then
If the transformations and , where is the maximum temperature which occurs at due to symmetry, are introduced
The above equation is nothing but Emden–Chandrasekhar equation,[14] which appears in astrophysics describing isothermal gas sphere. Unlike planar and cylindrical case, the spherical vessel has infinitely many solutions for oscillating about the point ,[15] instead of just two solutions, which was shown by Israel Gelfand.[16] The lowest branch will be chosen to explain explosive behavior.
From numerical solution, it is found that the critical Frank-Kamenetskii parameter is . The system has no steady state( or explodes) for and for , the system goes to a steady state with very slow reaction. The maximum temperature occurs at and maximum critical temperature is .
Non-symmetric geometries
For vessels which are not symmetric about the center (for example rectangular vessel), the problem involves solving a nonlinear partial differential equation instead of a nonlinear ordinary differential equation, which can be solved only through numerical methods in most cases. The equation is
with boundary condition on the bounding surfaces.
Applications
Since the model assumes homogeneous mixture, the theory is well applicable to study the explosive behavior of solid fuels (spontaneous ignition of bio fuels, organic materials, garbage, etc.,). This is also used to design explosives and fire crackers. The theory predicted critical values accurately for low conductivity fluids/solids with high conductivity thin walled containers.[17]
See also
References
- Frank-Kamenetskii, David A. "Towards temperature distributions in a reaction vessel and the stationary theory of thermal explosion." Doklady Akademii Nauk SSSR. Vol. 18. 1938.
- Frank-Kamenetskii, D. A. "Calculation of thermal explosion limits." Acta. Phys.-Chim USSR 10 (1939): 365.
- Semenov, N. N. "The calculation of critical temperatures of thermal explosion." Z Phys Chem 48 (1928): 571.
- Semenov, N. N. "On the theory of combustion processes." Z. phys. Chem 48 (1928): 571–582.
- Frank-Kamenetskii, David Albertovich. Diffusion and heat exchange in chemical kinetics. Princeton University Press, 2015.
- Linan, Amable, and Forman Arthur Williams. "Fundamental aspects of combustion." (1993).
- Williams, Forman A. "Combustion theory." (1985).
- Buckmaster, John David, and Geoffrey Stuart Stephen Ludford. Theory of laminar flames. Cambridge University Press, 1982.
- Buckmaster, John D., ed. The mathematics of combustion. Society for Industrial and Applied Mathematics, 1985.
- Zeldovich, I. A., Barenblatt, G. I., Librovich, V. B., and Makhviladze, G. M. (1985). Mathematical theory of combustion and explosions.
- Lewis, Bernard, and Guenther Von Elbe. Combustion, flames and explosions of gases. Elsevier, 2012.
- Chambre, P. L. "On the Solution of the Poisson‐Boltzmann Equation with Application to the Theory of Thermal Explosions." The Journal of Chemical Physics 20.11 (1952): 1795–1797.
- Lemke, H. (1913). Über die Differentialgleichungen, welche den Gleichgewichtszustand eines gasförmigem Himmelskörpers bestimmen, dessen Teile gegeneinander nach dem Newtonschen Gesetz gravitieren. Journal für die reine und angewandte Mathematik, 142, 118-145.
- Subrahmanyan Chandrasekhar. An introduction to the study of stellar structure. Vol. 2. Courier Corporation, 1958.
- Jacobsen, Jon, and Klaus Schmitt. "The Liouville–Bratu–Gelfand problem for radial operators." Journal of Differential Equations 184.1 (2002): 283–298.
- Gelfand, I. M. (1963). Some problems in the theory of quasilinear equations. Amer. Math. Soc. Transl, 29(2), 295–381.
- Zukas, Jonas A., William Walters, and William P. Walters, eds. Explosive effects and applications. Springer Science & Business Media, 2002.
External links
- The Frank-Kamenetskii problem in Wolfram solver http://demonstrations.wolfram.com/TheFrankKamenetskiiProblem/
- Tracking the Frank-Kamenetskii Problem in Wolfram solver http://demonstrations.wolfram.com/TrackingTheFrankKamenetskiiProblem/
- Planar solution in Chebfun solver http://www.chebfun.org/examples/ode-nonlin/BlowupFK.html