Frémy's salt
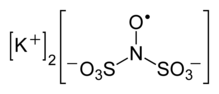
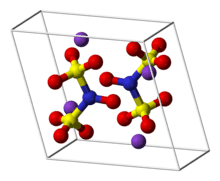
Frémy's salt is a chemical compound with the formula (K4[ON(SO3)2]2), sometimes written as (K2[NO(SO3)2]). It a bright yellowish-brown solid, but its aqueous solutions are bright violet.[1][2] The related sodium salt, i.e. disodium nitrosodisulfonate (NDS, Na2ON(SO3)2, CAS RN 29554-37-8) is also referred to as Frémy's salt.[3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Potassium nitrosodisulfonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.729 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| K2NO(SO3)2 | |
| Molar mass | 268.33 g/mol (potassium salt) |
| Hazards | |
| Main hazards | Harmful (Xn) |
| R-phrases (outdated) | R14 R20/21/22 |
| S-phrases (outdated) | S36 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Regardless of the cations, the salts are distinctive because aqueous solutions contain the radical [ON(SO3)2]2-.
Applications
Fremy's salt, being a long-lived free radical, is used as a standard in electron paramagnetic resonance (EPR) spectroscopy, e.g. for quantitation of radicals. It intense EPR spectrum is dominated by three lines of equal intensity with a spacing of about 13 G (= 1.3 mT).[4][5][6]
The inorganic aminoxyl group is a persistent radical, akin to TEMPO.
It has been used in some oxidation reactions, e.g. for oxidation of some anilines and phenols.[7][8][9][10][11] It can also be used as a model for peroxyl radicals in studies that examine the antioxidant mechanism of action in a wide range of natural products.[12]
Preparation
Fremy's salt is prepared from hydroxylaminedisulfonic acid. Oxidation of the conjugate base gives the purple dianion:
- HON(SO3H)2 → [HON(SO3)2]2- + 2 H+
- 2 [HON(SO3)2]2- + PbO2 → 2 [ON(SO3)2]2- + PbO + H2O
The synthesis can be performed by combining nitrite and bisulfite to give the hydroxylaminedisulfonate. Oxidation is typically conducted at low-temperature, either chemically or by electrolysis.[3][2]
Other reactions:
- HNO2 + 2 HSO3− → HON(SO3)22− + H2O
- 3 HON(SO3)22− + MnO4− + H+ → 3 ON(SO3)22− + MnO2 + 2 H2O
- 2 ON(SO3)22− + 4 K+ → K4[ON(SO3)2]2
History
The Frémy's salt was discovered in 1845 by Edmond Frémy (1814–1894).[13] Its use in organic synthesis was popularized by Hans Teuber, such that an oxidation using this salt is called the Teuber reaction.[9][10]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- http://chemistris.tripod.com/science/synthesis_of_fremys_salt.pdf
- Wehrli, Pius A.; Pigott, Foster (1972). "Oxidation with the nitrosodisulfonate radical. I. Preparation and use of sodium nitrosodisulfonate: trimethyl-p-benzoquinone". Organic Syntheses. 52: 83. doi:10.15227/orgsyn.052.0083.
- Wertz, J. E.; Bolton, J. R. (1972). Electron Spin Resonance: Elementary Theory and Practical Applications. New York: McGraw-Hill. ISBN 978-0-07-069454-5. - See page 463 for information on intensity measurements and page 86 for an EPR spectrum of Fremy's salt.
- Di Giulio; et al. (2000). "EPR study of Fremy's salt nitroxide reduction by ascorbic acid; influence of bulk pH values". Res. Chem. Intermed. 26 (9): 885–896. doi:10.1163/156856700X00372.
- Zielonka, Jacek; et al. (2005). "Mechanistic similarities between oxidation of hydroethidine by Fremy's salt and superoxide: Stopped-flow optical and EPR studies". Free Radical Biology & Medicine. 39 (7): 853–863. doi:10.1016/j.freeradbiomed.2005.05.001. PMID 16140206.
- Zimmer, Hans; Lankin, David C.; Horgan, Stephen W. (1971). "Oxidations with potassium nitrosodisulfonate (Fremy's radical). Teuber reaction". Chemical Reviews. 71 (2): 229–46. doi:10.1021/cr60270a005.CS1 maint: uses authors parameter (link)
- Islam, Imadul Islam; Skibo, Edward B.; Dorr, Robert T.; Alberts, David S. (1991). "Structure-activity studies of antitumor agents based on pyrrolo[1,2-a]benzimidazoles: new reductive alkylating DNA cleaving agents". Journal of Medicinal Chemistry. 34 (10): 2954–2961. doi:10.1021/jm00114a003. PMID 1920349.
- Teuber, Hans-J.; Benz, Siegfried (1967). "Reaktionen mit Nitrosodisulfonat, XXXVI. Chinolin-chinone-(5.6) aus 5-Hydroxy-chinolinen". Chem. Ber. 100 (9): 2918–29. doi:10.1002/cber.19671000916. Abstract (in German).
- Teuber, Hans-J. (1972). "Use of dipotassium nitrosodisulfonate (Fremy's salt): 4,5-dimethyl-o-benzoquinone". Org. Synth. 52: 88. doi:10.15227/orgsyn.052.0088.
- W. Xue; et al. (2002). "A metabolic activation mechanism of 7H-dibenzo[C,G]carbozole via 0-quinon. Part 1: synthesis of 7H-dibenzo[C,G]carbozole-3,4-dione and reactions with nucleophiles". Polycyclic Aromatic Compounds. 22 (3–4): 295–300. doi:10.1080/10406630290026957.
- Liu, Z.-L.; Han, Z.-X.; Chen, P.; Liu, Y.-C. (1990). "Stopped-flow ESR study on the reactivity of vitamin E, vitamin C and its lipophilic derivatives towards Fremy's salt in miceller systems". Chem. Phys. Lipids. 56 (1): 73–80. doi:10.1016/0009-3084(90)90090-E. PMID 1965427.
- See:
- Frémy, E. (1845) "Sur un nouvelle série d'acides formés d'oxygène, de soufre, d'hydrogène et de d'azote" (On a new series of acids formed from oxygen, sulfur, hydrogen, and nitrogen), Annales de Chimie et de Physique, 3rd series, 15 : 408-488. Frémy's salt appears on p. 447, where it's called "sulfazidate de potasse".
- Frémy, E. (1845) "Sur un nouvelle série d'acides formés d'oxygène, de soufre, d'hydrogène et de d'azote" (On a new series of acids formed from oxygen, sulfur, hydrogen, and nitrogen), Comptes rendus, 21 : 218-226. This is a condensed version of the article that appeared in Annales de Chimie et de Physique.
- "Séances académiques," L'Institut, no. 604, 23 July 1845, pp. 265-266.
- "Séances académiques," L'Institut, no. 619, 12 November 1845, pp. 393. Here a committee of the French Academy of Sciences reviewed Frémy's findings.
- Edward Divers and Tamemasa Haga (1900) "Identification and constitution of Fremy's sulphazotised salts of potassium," Journal of the Chemical Society, Transactions, 77 : 440-446. doi:10.1039/CT9007700440 Here, correct formulae for Frémy's salts are presented. On p. 445, the salt that Frémy called sulfazidate is identified as ON(SO3K)2.
Further reading
- Morey, J. (1988). "Undergraduate Experiments with a Long-Lived Radical (Fremy's salt): Synthesis of 1,4-Benzoquinones by Degradative Oxidation of p-Hydroxybenzyl Alcohols". J. Chem. Educ. 65 (7): 627–629. doi:10.1021/ed065p627.