10-Formyltetrahydrofolate
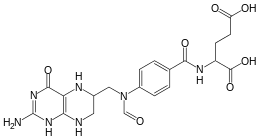
10-Formyltetrahydrofolate (10-CHO-THF) is a form of tetrahydrofolate that acts as a donor of formyl groups in anabolism. In these reactions 10-CHO-THF is used as a substrate in formyltransferase reactions.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-{[4-[(2-amino-4-oxo-5,6,7,8-tetrahydro-1H-pteridin-6-yl) | |
| Other names
10-CHO-THF 10-formylH4folate N10-formyltetrahydrofolate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | 10-formyl-tetrahydrofolate |
PubChem CID |
|
| |
| |
| Properties | |
| C20H23N7O7 | |
| Molar mass | 473.44 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Functions
Two equivalents of 10-CHO-THF are required in purine biosynthesis through the pentose phosphate pathway, where 10-CHO-THF is a substrate for phosphoribosylaminoimidazolecarboxamide formyltransferase.
10-CHO-THF is required for the formylation of methionyl-tRNA formyltransferase to give fMet-tRNA.[1]
Formation from methenyltetrahydrofolate
10-CHO-THF is produced from methylenetetrahydrofolate (CH2H4F) via a two step process. The first step generates 5,10-methenyltetrahydrofolate:[2]
- CH2H4F + NAD+ CH2H2F + NADH + H+
In the second step 5,10-methenyltetrahydrofolate undergoes hydrolysis:
- CH2H2F + H2O CHO-H4F +
The latter is equivalently written:
- 5,10-methenyltetrahydrofolate + H2O 10-formyltetrahydrofolate
10-CHO-THF is also produced by the reaction
- ATP + formate + tetrahydrofolate ADP + phosphate + 10-formyltetrahydrofolate
This reaction is catalyzed by formate-tetrahydrofolate ligase.
It can be converted back into tetrahydrofolate (THF) by formyltetrahydrofolate dehydrogenase or THF and formate by formyltetrahydrofolate deformylase.
References
- Voet, Donald (2016). Fundamentals of Biochemistry: Life at the Molecular Level (5th ed.). Wiley. pp. 1006–1007. ISBN 978-1-118-91840-1.
- Peter D. Pawelek; Robert E. MacKenzie (1998). "Methenyltetrahydrofolate Cyclohydrolase Is Rate Limiting for the Enzymatic Conversion of 10-Formyltetrahydrofolate to 5,10-Methylenetetrahydrofolate in Bifunctional Dehydrogenase-Cyclohydrolase Enzymes". Biochemistry. 37: 1109–1115. doi:10.1021/bi971906t. PMID 9454603.