Folin's reagent
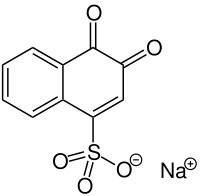
Folin's reagent or sodium 1,2-naphthoquinone-4-sulfonate is a chemical reagent used as a derivatizing agent to measure levels of amines and amino acids.[1] The reagent reacts with them in alkaline solution to produce a fluorescent material that can be easily detected.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium 3,4-dioxo-3,4-dihydronaphthalene-1-sulfonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.555 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H5NaO5S | |
| Molar mass | 260.20 g/mol |
| Hazards | |
| Safety data sheet | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
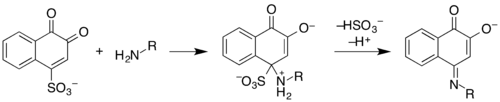
This should not be confused with Folin-Ciocalteu reagent, that is used to detect phenolic compounds.
The Folin reagent can be used with an acidic secondary reagent to distinguish MDMA and related compounds from PMMA and related compounds.[3]
| Substance | Reaction |
|---|---|
| MDMA | Pink[3] |
| MDE | No Reaction[3] |
| MDA | Yellow[3] |
| BZP | Orange to red[3] |
| TFMPP | Pinkish red[3] |
| Methylone | No reaction[3] |
| Methamphetamine | Pink[3] |
| Amphetamine | Light orange[3] |
| PMA | Light Yellow[3] |
| PMMA | Light orange[3] |
| Ketamine | No reaction[3] |
| Mescaline | No reaction[3] |
| Cocaine | No reaction[3] |
| Aspirin | No reaction[3] |
| Sugar | No reaction[3] |
See also
References
- Saurina J, Hernández-Cassou S (1994). "Determination of amino acids by ion-pair liquid chromatography with post-column derivatization using 1,2-naphthoquinone-4-sulfonate". Journal of Chromatography A. 676 (2): 311–9. doi:10.1016/0021-9673(94)80431-1. PMID 7921184.
- Kobayashi Y, Kubo H, Kinoshita T (1987). "Fluorometric determination of guanidino compounds by new postcolumn derivatization system using reversed-phase ion-pair high-performance liquid chromatography". Anal. Biochem. 160 (2): 392–8. doi:10.1016/0003-2697(87)90066-2. PMID 3578768.
- "Folin Reagent Testing Kit". Dancesafe. June 2015. Archived from the original on 11 September 2015. Retrieved 8 August 2015.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.