Ferromanganese
Ferromanganese, a ferroalloy with high content of manganese, is made by heating a mixture of the oxides MnO2 and Fe2O3, with carbon, usually as coal and coke, in either a blast furnace or an electric arc furnace-type system, called a submerged arc furnace. The oxides undergo carbothermal reduction in the furnaces, producing the ferromanganese. Ferromanganese is used as a deoxidizer for steel.

Henry Bessemer invented the use of ferromanganese as a method of introducing manganese in controlled proportions during the production of steel. The advantage of combining powdered iron oxide and manganese oxide together is the lower melting point of the combined alloy compared to pure manganese oxide.
A North American standard specification is ASTM A99. The ten grades covered under this specification includes;
- Standard ferromanganese
- Medium-carbon ferromanganese
- Low-carbon ferromanganese
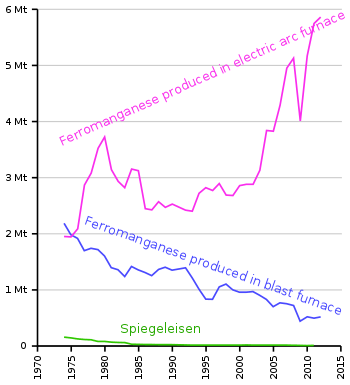
A similar material is a pig iron with high content of manganese, is called spiegeleisen.
History
In 1872, Lambert von Pantz produced ferromanganese in a blast furnace, with significantly higher manganese content than was previously possible (37% instead of the previous 12%). This won his company international recognition, including a gold medal at the 1873 World Exposition in Vienna and a certificate of award at the 1876 Centennial Exposition in Pennsylvania.[1][2]
References
- Hočevar, Toussaint (1965). The structure of the Slovenian economy, 1848-1963. Studia Slovenica. p. 30. COBISS 26847745.
- Vilman, Vladimir (2004). "Von Pantzove gravitacijske žičnice na Slovenskem" [Von Pantnz's gravity ropeways in Slovenia]. Mednarodno posvetovanje Spravilo lesa z žičnicami za trajnostno gospodarjenje z gozdovi [International Symposium Cable Yarding Suitable for Sustainable Forest Management] (PDF) (in Slovenian). pp. 9–33. Archived from the original (PDF) on 2014-04-07. Retrieved 2014-04-03.
Further reading
- Jorgenson, John D.; Corathers, Lisa A.; Gambogi, Joseph; Kuck, Peter H.; Magyar, Michael J.; Papp, John F.; Shedd, Kim B. "Minerals Yearbook 2006: Ferroalloys" (PDF). United States Geological Survey. Retrieved 2009-04-24.