Farinomalein
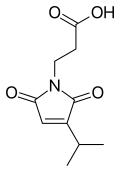
Farinomalein is a natural maleimide with formula C10H13NO4 - was first isolated from the entomopathogenic fungus Isaria farinosa (Paecilomyces farinosus) - source H599 (Japan).[1]
 | |
| Names | |
|---|---|
| IUPAC name
3-(2,5-Dioxo-3-propan-2-ylpyrrol-1-yl)propanoic acid | |
| Systematic IUPAC name
3-(3-Isopropyl-2,5-dioxo-pyrrol-1-yl)propanoic acid | |
| Other names
2,5-Dihydro-3-(1-methylethyl)-2,5-dioxo-1H-pyrrole-1-propanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H13NO4 | |
| Molar mass | 211.217 g·mol−1 |
| Appearance | White powder |
| Melting point | 75 to 77 °C (167 to 171 °F; 348 to 350 K) |
| Solubility | CH2Cl2, acetone, toluene, CH3OH |
| Vapor pressure | 0 mmHg (25 °C) |
| Hazards | |
| R-phrases (outdated) | R43 |
| S-phrases (outdated) | S22-S24-S37-S61 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Farinomalein has shown potent and selective inhibition (0.15-5 μg/disk) against eight isolates of plant pathogenic Phytophthora sojae.[2] These results suggest that farinomalein might be useful as a candidate pesticide for the treatment of Phytophthora stem rot in soybean.[2]
Synthesis
A simple two-stage synthesis from the γ-hydroxybutenolide compound, 5-hydroxy-4-methyl-2-5(H)-furanone, has been reported.[3] Firstly, the furanone is oxidized to 3-isopropylfuran-2,5-dione by Dess–Martin periodinane, followed by acetic acid reflux with beta-alanine. The white powdered product has a melting point of 75-77 °C.
References
- Putri, Sastia P; Kinoshita, Hiroshi; Ihara, Fumio; Igarashi, Yasuhiro; Nihira, Takuya (2009). "Farinomalein, a Maleimide-Bearing Compound from the Entomopathogenic Fungus Paecilomyces farinosus". Journal of Natural Products. 72 (8): 1544–6. doi:10.1021/np9002806. PMID 19670877.
- Sastia Prama Putri, Hiroshi Kinoshita, Masayasu Kato and Takuya Nihira. Antimicrobial and antioomycete activities of the novel antibiotic farinomalein. Poster Presentation 2P-2124, Annual Conference, The Society for Bioscience and Bioengineering, Japan, 28 October 2010.
- Miles, William H; Yan, Ming (2010). "Synthesis of farinomalein". Tetrahedron Letters. 51 (13): 1710. doi:10.1016/j.tetlet.2010.01.083.