Exometabolomics
Exometabolomics, also known as 'metabolic footprinting',[1][2] is the study of extracellular metabolites and is a sub-field of metabolomics.[3]
While the same analytical approaches used for profiling metabolites apply to exometabolomics, including liquid-chromatography mass spectrometry (LC-MS), nuclear magnetic resonance (NMR) and gas chromatography–mass spectrometry (GC–MS), analysis of exometabolites provides specific challenges and is most commonly focused on investigation of the transformations of exogenous metabolite pools by biological systems.[3] Typically, these experiments are performed by comparing metabolites at two or more time points, for example, spent vs. uninoculated/control culture media; this approach can differentiate different physiological states of wild-type yeast and between yeast mutants.[1] Since, in many cases, the exometabolite (extracellular) pool is less dynamic than endometabolite (intracellular) pools (which are often perturbed during sample processing) and chemically defined media can be used, it reduces some of the experimental challenges of metabolomics.[4]
Exometabolomics is also used as a complementary tool with genomic, transcriptomic[5] and proteomic data, to gain insight into the function of genes and pathways. Additionally, exometabolomics can be used to measure polar molecules being consumed or released by an organism, and to measure secondary metabolite production.[6][7]
History
The study of extracellular metabolites has been prevalent in scientific literature.[8][9][10] However, global exometabolite profiling was only realized with recent advances allowing for improved chromatographic separation and detection of hundreds to thousands of compounds by the mid-2000s.[7] The first work to demonstrate the biological relevance of comparative profiling of exometabolite pools was not until 2003, when the term "metabolite footprinting" was coined by Jess Allen and coworkers.[1][7] This work attracted a great deal of interest in the community, particularly for characterization of microbial metabolism.[2] The idea of the "exometabolome" encompassing the components of the exometabolite pool was not introduced until 2005.[11]
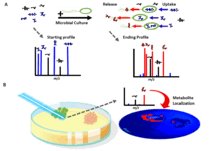
Recent advances in mass spectrometry imaging have allowed for spatial localization of released metabolites.[12] As the field of microbiology becomes increasingly more centered on microbial community structure, exometabolomics has provided for rapid understanding of metabolic interactions between two or more species.[13] Recently, exometabolomics has been used to design co-culture systems.[14] Because the analysis of extracellular metabolites allows for the predictions and determinations of metabolite exchange, exometabolomics analyses can be used for understanding community ecological networks.[15]
Analytical technologies
In principle, any technologies used for metabolomics can be used for exometabolomics. However, liquid chromatography–mass spectrometry (LC–MS) has been the most widely used.[3] As with typical metabolomic measurements, metabolites are identified based on accurate mass, retention time, and their MS/MS fragmentation patterns, in comparison to authentic standards. Chromatographies typically used are hydrophilic interaction liquid chromatography for the measurement of polar metabolites,[16] or reversed-phase (C18) chromatography for the measurement of non-polar compounds, lipids, and secondary metabolites.[17] Gas chromatography–mass spectrometry can also be used to measure sugars and other carbohydrates, and to obtain complete metabolic profiles.[18]
Because LC–MS does not give spatial data on metabolite localization, it can be complemented with mass spectrometry imaging (MSI).[3]
Applications
Exometabolomic techniques have been used in the following fields:
Functional genomics
Metabolite utilization to annotate function of unknown genes.[19]
Bioenergy
In lignocellulosic feedstock studies.[20]
Agriculture and food
Characterization of plant root exometabolites to determine how exometabolites affect Plant-growth promoting rhizobacteria.[21]
Metabolic footprinting of yeast strains for identification of yeast strains optimal for enhancing fermentation performance and positive attributes in wine.[22]
Health
Differentiating healthy versus cancerous bladder cells with metabolic footprinting.[23]
Footprinting, in combination with other techniques, for early recognition of outbreak and strain characterization.[24]
Studying aging with C. elegans exometabolomics.[25]
Extracellular metabolite analysis to evaluate pathogenic mechanism of intracellular protozoal parasite.[26]
Analysis of carbon cycling
Global carbon fixation, phytoplankton/dinoflaggelate interactions, and exometabolomics.[27]
Microbial communities
Interaction of E. coli exometabolites with C. elegans affects life span.[28]
Bacteria and yeast in dairy systems.[13]
Bioremediation
Metabolic niche partitioning
In 2010, exometabolomics analysis of the cyanobacterium, Synechococcus sp. PCC 7002 by Baran, et al. revealed that this photoautotroph could deplete a diverse pool of exogenous metabolites.[29] A follow-up exometabolomics study on sympatric microbial isolates from biological soil crust, which exist in communities with cyanobacteria in the desert soils of the Colorado Plateau, suggested that metabolite niche partitioning exists in these communities, where each isolate only utilizes 13-26% of metabolites from the soil [30]
Secondary metabolites
Metabolic footprinting for determination of antifungal substances' mode of action[31]
See also
- Mass spectrometry
- Metabolomics
- Metabolome
- Metabolite fingerprinting
- Mass spectrometry imaging
References
- Allen, Jess (2003). "High-throughput classification of yeast mutants for functional genomics using metabolic footprinting". Nature Biotechnology. 21 (6): 692–696. doi:10.1038/nbt823. PMID 12740584.
- Mapelli, Valeria; Olsson, Lisbeth; Nielsen, Jens (2008-09-01). "Metabolic footprinting in microbiology: methods and applications in functional genomics and biotechnology". Trends in Biotechnology. 26 (9): 490–497. doi:10.1016/j.tibtech.2008.05.008. ISSN 0167-7799. PMID 18675480.
- Silva, Leslie; Northen, Trent (2015). "Exometabolomics and MSI: deconstructing how cells interact to transform their small molecule environment". Current Opinion in Biotechnology. 34: 209–216. doi:10.1016/j.copbio.2015.03.015. PMID 25855407.
- Cuperlovic-Culf, Miroslava (2014). "Metabolomics in Animal Cell Culture". In Al-Rubeai, M. (ed.). Animal Cell Culture. Springer International Publishing. p. 628. ISBN 978-3-319-10320-4.
- Rossouw, Debra; Næs, Tormod; Bauer, Florian F. (2008-01-01). "Linking gene regulation and the exo-metabolome: A comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast". BMC Genomics. 9: 530. doi:10.1186/1471-2164-9-530. ISSN 1471-2164. PMC 2585593. PMID 18990252.
- Chumnanpuen, Pramote (2014). "Dynamic Metabolic Footprinting Reveals the Key Components of Metabolic Network in Yeast Saccharomyces cerevisiae". International Journal of Genomics. 2014: 894296. doi:10.1155/2014/894296. PMC 3926413. PMID 24616891.
- Kell, Douglas B.; Brown, Marie; Davey, Hazel M.; Dunn, Warwick B.; Spasic, Irena; Oliver, Stephen G. (2005-07-01). "Metabolic footprinting and systems biology: the medium is the message". Nature Reviews Microbiology. 3 (7): 557–565. doi:10.1038/nrmicro1177. ISSN 1740-1526. PMID 15953932.
- Vavilova, N. A.; Ustinova, M. V.; Voinova, T. M.; Stepanichenko, N. N.; Ten, L. N.; Mukhamedzhanov, S. Z.; Dzhavakhiya, V. G. (1988-07-01). "Polyketide exometabolites of the causative agent of rice blast and their role in pathogenesis". Chemistry of Natural Compounds. 24 (4): 487–491. doi:10.1007/BF00598539. ISSN 0009-3130.
- Tambiev, A. H.; Shelyastina, N. N.; Kirikova, N. N. (1989-01-01). "Exometabolites of Lipid Nature from Two Species of Marine Microalgae". Functional Ecology. 3 (2): 245–247. doi:10.2307/2389307. JSTOR 2389307.
- Badenoch-Jones, Jane; Summons, R. E.; Djordjevic, M. A.; Shine, J.; Letham, D. S.; Rolfe, B. G. (1982-08-01). "Mass Spectrometric Quantification of Indole-3-Acetic Acid in Rhizobium Culture Supernatants: Relation to Root Hair Curling and Nodule Initiation". Applied and Environmental Microbiology. 44 (2): 275–280. ISSN 0099-2240. PMC 242007. PMID 16346073.
- Nielsen, Jens; Oliver, Stephen (2005-11-01). "The next wave in metabolome analysis". Trends in Biotechnology. 23 (11): 544–546. doi:10.1016/j.tibtech.2005.08.005. ISSN 0167-7799. PMID 16154652.
- Louie, Katherine B.; Bowen, Benjamin P.; Cheng, Xiaoliang; Berleman, James E.; Chakraborty, Romy; Deutschbauer, Adam; Arkin, Adam; Northen, Trent R. (2013-11-19). ""Replica-Extraction-Transfer" Nanostructure-Initiator Mass Spectrometry Imaging of Acoustically Printed Bacteria". Analytical Chemistry. 85 (22): 10856–10862. doi:10.1021/ac402240q. ISSN 0003-2700. PMID 24111681.
- Honoré, Anders H.; Thorsen, Michael; Skov, Thomas (2013-08-18). "Liquid chromatography–mass spectrometry for metabolic footprinting of co-cultures of lactic and propionic acid bacteria". Analytical and Bioanalytical Chemistry. 405 (25): 8151–8170. doi:10.1007/s00216-013-7269-3. ISSN 1618-2642. PMID 23954995.
- Kosina, Suzanne M.; Danielewicz, Megan A.; Mohammed, Mujahid; Ray, Jayashree; Suh, Yumi; Yilmaz, Suzan; Singh, Anup K.; Arkin, Adam P.; Deutschbauer, Adam M. (2016-02-17). "Exometabolomics Assisted Design and Validation of Synthetic Obligate Mutualism". ACS Synthetic Biology. 5 (7): 569–576. doi:10.1021/acssynbio.5b00236. PMID 26885935.
- Feist, Adam M.; Herrgard, Markus J. (2008). "Reconstruction of biochemical networks in microorganisms". Nature Reviews Microbiology. 7 (2): 129–143. doi:10.1038/nrmicro1949. PMC 3119670. PMID 19116616.
- McNamara, L. E.; Sjostrom, T.; Meek, R. M. D.; Oreffo, R. O. C.; Su, B.; Dalby, M. J.; Burgess, K. E. V. (2012). "Metabolomics: a valuable tool for stem cell monitoring in regenerative medicine". Journal of the Royal Society Interface. 9 (73): 1713–1724. doi:10.1098/rsif.2012.0169. PMC 3385772. PMID 22628210.
- Gao, Peng; Xu, Guowang (2014). "Mass-spectrometry-based microbial metabolomics: recent developments and applications". Anal Bioanal Chem. 407 (3): 669–680. doi:10.1007/s00216-014-8127-7. PMID 25216964.
- Sue, T.; Obolonkin, V.; Griffiths, H.; Villas-Boas, S. G. (2011). "An Exometabolomics Approach to Monitoring Microbial Contamination in Microalgal Fermentation Processes by Using Metabolic Footprint Analysis". Applied and Environmental Microbiology. 77 (21): 7605–7610. doi:10.1128/aem.00469-11. PMC 3209156. PMID 21890679.
- Baran, Richard; Bowen, Benjamin P.; Price, Morgan N.; Arkin, Adam P.; Deutschbauer, Adam M.; Northen, Trent R. (2013-01-18). "Metabolic Footprinting of Mutant Libraries to Map Metabolite Utilization to Genotype". ACS Chemical Biology. 8 (1): 189–199. doi:10.1021/cb300477w. ISSN 1554-8929. PMID 23082955.
- Zha, Ying; Punt, Peter J. (2013-02-11). "Exometabolomics Approaches in Studying the Application of Lignocellulosic Biomass as Fermentation Feedstock". Metabolites. 3 (1): 119–143. doi:10.3390/metabo3010119. PMC 3901257. PMID 24957893.
- Kravchenko, L. V.; Azarova, T. S.; Leonova-Erko, E. I.; Shaposhnikov, A. I.; Makarova, N. M.; Tikhonovich, I. A. (2003-01-01). "Root Exudates of Tomato Plants and Their Effect on the Growth and Antifungal Activity of Pseudomonas Strains". Microbiology. 72 (1): 37–41. doi:10.1023/A:1022269821379. ISSN 0026-2617.
- Richter, Chandra L.; Dunn, Barbara; Sherlock, Gavin; Pugh, Tom (2013-06-01). "Comparative metabolic footprinting of a large number of commercial wine yeast strains in Chardonnay fermentations". FEMS Yeast Research. 13 (4): 394–410. doi:10.1111/1567-1364.12046. ISSN 1567-1364. PMID 23528123.
- Pasikanti, Kishore Kumar; Norasmara, Juwita; Cai, Shirong; Mahendran, Ratha; Esuvaranathan, Kesavan; Ho, Paul C.; Chan, Eric Chun Yong (2010-08-05). "Metabolic footprinting of tumorigenic and nontumorigenic uroepithelial cells using two-dimensional gas chromatography time-of-flight mass spectrometry". Analytical and Bioanalytical Chemistry. 398 (3): 1285–1293. doi:10.1007/s00216-010-4055-3. ISSN 1618-2642. PMID 20686754.
- To, Kelvin K. W.; Fung, Ami M. Y.; Teng, Jade L. L.; Curreem, Shirly O. T.; Lee, Kim-Chung; Yuen, Kwok-Yung; Lam, Ching-Wan; Lau, Susanna K. P.; Woo, Patrick C. Y. (2012-11-07). "Tsukamurella pseudo-outbreak characterized by phenotypic tests, 16S rRNA sequencing, pulsed-field gel electrophoresis and metabolic footprinting". Journal of Clinical Microbiology. 51 (1): JCM.02845–12. doi:10.1128/JCM.02845-12. ISSN 0095-1137. PMC 3536211. PMID 23135942.
- Mishur, RobertJ.; Butler, JeffreyA.; Rea, ShaneL. (2013-01-01). Tollefsbol, Trygve O. (ed.). Exometabolomic Mapping of Caenorhabditis elegans: A Tool to Noninvasively Investigate Aging. Methods in Molecular Biology. 1048. Humana Press. pp. 195–213. doi:10.1007/978-1-62703-556-9_15. ISBN 9781627035552. PMID 23929107.
- Elsheikha, Hany M; Alkurashi, Mamdowh; Kong, Kenny; Zhu, Xing-Quan (2014-06-28). "Metabolic footprinting of extracellular metabolites of brain endothelium infected with Neospora caninum in vitro". BMC Research Notes. 7 (1): 406. doi:10.1186/1756-0500-7-406. PMC 4105892. PMID 24973017.
- Poulson-Ellestad, Kelsey L.; Harvey, Elizabeth L.; Johnson, Matthew D.; Mincer, Tracy J. (2016-01-01). "Evidence for Strain-Specific Exometabolomic Responses of the Coccolithophore Emiliania huxleyi to Grazing by the Dinoflagellate Oxyrrhis marina". Frontiers in Marine Science. 3: 1. doi:10.3389/fmars.2016.00001.
- 1989-, Correia, Gonçalo dos Santos. "Coupling metabolic footprinting and flux balance analysis to predict how single gene knockouts perturb microbial metabolism". repositorio.ul.pt. Retrieved 2016-04-23.CS1 maint: numeric names: authors list (link)
- Baran, Richard; Bowen, Benjamin P.; Northen, Trent R. (2011-01-12). "Untargeted metabolic footprinting reveals a surprising breadth of metabolite uptake and release by Synechococcus sp. PCC 7002". Molecular BioSystems. 7 (12): 3200–3206. doi:10.1039/C1MB05196B. PMID 21935552.
- Baran, Richard; Brodie, Eoin L.; Mayberry-Lewis, Jazmine; Hummel, Eric; Da Rocha, Ulisses Nunes; Chakraborty, Romy; Bowen, Benjamin P.; Karaoz, Ulas; Cadillo-Quiroz, Hinsby (2015-09-22). "Exometabolite niche partitioning among sympatric soil bacteria". Nature Communications. 6: 8289. Bibcode:2015NatCo...6.8289B. doi:10.1038/ncomms9289. PMC 4595634. PMID 26392107.
- Allen, Jess; Davey, Hazel M.; Broadhurst, David; Rowland, Jem J.; Oliver, Stephen G.; Kell, Douglas B. (2004-10-01). "Discrimination of Modes of Action of Antifungal Substances by Use of Metabolic Footprinting". Applied and Environmental Microbiology. 70 (10): 6157–6165. doi:10.1128/AEM.70.10.6157-6165.2004. ISSN 0099-2240. PMC 522091. PMID 15466562.