Epigenome editing
Epigenome editing or Epigenome engineering is a type of genetic engineering in which the epigenome is modified at specific sites using engineered molecules targeted to those sites (as opposed to whole-genome modifications). Whereas gene editing involves changing the actual DNA sequence itself, epigenetic editing involves modifying and presenting DNA sequences to proteins and other DNA binding factors that influence DNA function. By "editing” epigenomic features in this manner, researchers can determine the exact biological role of an epigenetic modification at the site in question.
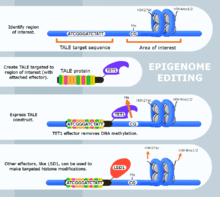
The engineered proteins used for epigenome editing are composed of a DNA binding domain that target specific sequences and an effector domain that modifies epigenomic features. Currently, three major groups of DNA binding proteins have been predominantly used for epigenome editing: Zinc finger proteins, Transcription Activator-Like Effectors (TALEs) and nuclease deficient Cas9 fusions (CRISPR).
General concept
Comparing genome-wide epigenetic maps with gene expression has allowed researchers to assign either activating or repressing roles to specific modifications. The importance of DNA sequence in regulating the epigenome has been demonstrated by using DNA motifs to predict epigenomic modification.[1] Further insights into mechanisms behind epigenetics have come from in vitro biochemical and structural analyses. Using model organisms, researchers have been able to describe the role of many chromatin factors through knockout studies. However knocking out an entire chromatin modifier has massive effects on the entire genome, which may not be an accurate representation of its function in a specific context. As one example of this, DNA methylation occurs at repeat regions, promoters, enhancers, and gene bodies. Although DNA methylation at gene promoters typically correlates with gene repression, methylation at gene bodies is correlated with gene activation, and DNA methylation may also play a role in gene splicing.[2] The ability to directly target and edit individual methylation sites is critical to determining the exact function of DNA methylation at a specific site. Epigenome editing is a powerful tool that allows this type of analysis. For site-specific DNA methylation editing as well as for histone editing, genome editing systems have been adapted into epigene editing systems. In short, genome homing proteins with engineered or naturally occurring nuclease functions for gene editing, can be mutated and adapted into purely delivery systems. An epigenetic modifying enzyme or domain can be fused to the homing protein and local epigenetic modifications can be altered upon protein recruitment.
Targeting proteins
TALE
The Transcription Activator-Like Effector (TALE) protein recognizes specific DNA sequences based on the composition of its DNA binding domain.[3] This allows the researcher to construct different TALE proteins to recognize a target DNA sequence by editing the TALE's primary protein structure. The binding specificity of this protein is then typically confirmed using Chromatin Immunoprecipitation (ChIP) and Sanger sequencing of the resulting DNA fragment.[4][5][6] This confirmation is still required on all TALE sequence recognition research.[7] When used for epigenome editing, these DNA binding proteins are attached to an effector protein. Effector proteins that have been used for this purpose include Ten-eleven translocation methylcytosine dioxygenase 1 (TET1),[5] Lysine (K)-specific demethylase 1A (LSD1)[6] and Calcium and integrin binding protein 1 (CIB1).[4]
Zinc finger proteins
The use of zinc finger-fusion proteins to recognize sites for epigenome editing has been explored as well. Maeder et al. has constructed a ZF-TET1 protein for use in DNA demethylation.[5] These zinc finger proteins work similarly to TALE proteins in that they are able to bind to sequence specific sites in on the DNA based on their protein structure which can be modified. Chen et al. have successfully used a zinc finger DNA binding domain coupled with the TET1 protein to induce demethylation of several previously silenced genes.[8]
CRISPR-Cas
The Clustered Regulatory Interspaced Short Palindromic Repeat (CRISPR)-Cas system functions as a DNA site-specific nuclease.[9] In the well-studied type II CRISPR system, the Cas9 nuclease associates with a chimera composed of tracRNA and crRNA. This chimera is frequently referred to as a guide RNA (gRNA). When the Cas9 protein associates with a DNA region-specific gRNA, the Cas9 cleaves DNA at targeted DNA loci. However, when the D10A and H840A point mutations are introduced, a catalytically-dead Cas9 (dCas9) is generated that can bind DNA but will not cleave.[10] The dCas9 system has been utilized for targeted epigenetic reprogramming in order to introduce site-specific DNA methylation. By fusing the DNMT3a catalytic domain with the dCas9 protein, dCas9-DNMT3a is capable of achieving targeted DNA methylation of a targeted region as specified by the present guide RNA.[11] Similarly, dCas9 has been fused with the catalytic core of the human acetyltransferase p300. dCas9-p300 successfully catalyzes targeted acetylation of histone H3 lysine 27.[12]
A variant in CRISPR epigenome editing (called FIRE-Cas9) allows to reverse the changes made, in case something went wrong.[13][14]
Commonly used effector proteins
TET1 induces demethylation of cytosine at CpG sites. This protein has been used to activate genes that are repressed by CpG methylation and to determine the role of individual CpG methylation sites.[5] LSD1 induces the demethylation of H3K4me1/2, which also causes an indirect effect of deacetylation on H3K27. This effector can be used on histones in enhancer regions, which can changes the expression of neighboring genes.[6] CIB1 is a light sensitive cryptochrome, this cryptochrome is fused to the TALE protein. A second protein contains an interaction partner (CRY2) fused with a chromatin/DNA modifier (ex. SID4X). CRY2 is able to interact with CIB1 when the cryptochrome has been activated by illumination with blue light.[15] The interaction allows the chromatin modifier to act on the desired location. This means that the modification can be performed in an inducible and reversible manner, which reduces long-term secondary effects that would be caused by constitutive epigenetic modification.[4]
Applications
Studying enhancer function and activity
Editing of gene enhancer regions in the genome through targeted epigenetic modification has been demonstrated by Mendenhall et al. (2013).[6] This study utilized a TALE-LSD1 effector fusion protein in order to target enhancers of genes, to induce enhancer silencing in order to deduce enhancer activity and gene control. Targeting specific enhancers followed by locus specific RT-qPCR allows for the genes affected by the silenced enhancer to be determined. Alternatively, inducing enhancer silencing in regions upstream of genes allows for gene expression to be altered. RT-qPCR can then be utilized to study effects of this on gene expression. This allows for enhancer function and activity to be studied in detail.[6]
Determining the function of specific methylation sites
It is important to understand the role specific methylation sites play regulating in gene expression. To study this, one research group used a TALE-TET1 fusion protein to demethylate a single CpG methylation site.[5] Although this approach requires many controls to ensure specific binding to target loci, a properly performed study using this approach can determine the biological function of a specific CpG methylation site.[5]
Determining the role of epigenetic modifications directly
Epigenetic editing using an inducible mechanism offers a wide array of potential use to study epigenetic effects in various states. One research group employed an optogenetic two-hybrid system which integrated the sequence specific TALE DNA-binding domain with a light-sensitive cryptochrome 2 protein (CIB1).[4] Once expressed in the cells, the system was able to inducibly edit histone modifications and determine their function in a specific context.[4]
Limitations
Sequence specificity is critically important in epigenome editing and must be carefully verified (this can be done using chromatin immunoprecipitation followed by Sanger sequencing to verify the targeted sequence).[7] It is unknown if the TALE fusion may cause effects on the catalytic activity of the epigenome modifier. This could be especially important in effector proteins that require multiple subunits and complexes such as the Polycomb repressive complex.[7] Proteins used for epigenome editing may obstruct ligands and substrates at the target site.[7] The TALE protein itself may even compete with transcription factors if they are targeted to the same sequence.[7] In addition, DNA repair systems could reverse the alterations on the chromatin and prevent the desired changes from being made.[7] It is therefore necessary for fusion constructs and targeting mechanisms to be optimized for reliable and repeatable epigenome editing.
References for further reading
- Srivastava, D.; DeWitt, N. (2016). "In Vivo Cellular Reprogramming: The Next Generation". Cell. 166 (6): 1386–1396. doi:10.1016/j.cell.2016.08.055. PMC 6234007. PMID 27610565.
- Chakraborty, S.; Ji, H.; Kabadi, A. M.; Gersbach, C. A.; Christoforou, N.; Leong, K. W. (2014). "A CRISPR/Cas9-based system for reprogramming cell lineage specification". Stem Cell Reports. 3 (6): 940–947. doi:10.1016/j.stemcr.2014.09.013. PMC 4264059. PMID 25448066.
- Thakore, P. I.; Black, J. B.; Hilton, I. B.; Gersbach, C. A. (2016). "Editing the epigenome: technologies for programmable transcription and epigenetic modulation". Nature Methods. 13 (2): 127–137. doi:10.1038/nmEth.3733. PMC 4922638. PMID 26820547.
- Vora, S.; Tuttle, M.; Cheng, J.; Church, G. (2016). "Next stop for the CRISPR revolution: RNA guided epigenetic regulators". The FEBS Journal. 283 (17): 3181–3193. doi:10.1111/febs.13768. PMID 27248712.
- Nelson, C. E.; Gersbach, C. A. (2016). "Engineering Delivery Vehicles for Genome Editing". Annual Review of Chemical and Biomolecular Engineering. 7: 637–662. doi:10.1146/annurev-chembioeng-080615-034711. PMID 27146557.
- Hilton, I. B.; D'Ippolito, A. M.; Vockley, C. M.; Thakore, P. I.; Crawford, G. E.; Reddy, T. E.; Gersbach, C. A. (2015). "Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers". Nature Biotechnology. 33 (5): 510–517. doi:10.1038/nbt.3199. PMC 4430400. PMID 25849900.
- McDonald, J. I.; Celik, H.; Rois, L. E.; Fishberger, G.; Fowler, T.; Rees, R.; Challen, G. A. (2016). "Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation". Biology Open. 5 (6): 866–874. doi:10.1242/bio.019067. PMC 4920199. PMID 27170255.
- Xu, X.; Tao, Y.; Gao, X.; Zhang, L.; Li, X.; Zou, W.; Hu, R. (2016). "A CRISPR-based approach for targeted DNA demethylation". Cell Discovery. 2: 16009. doi:10.1038/celldisc.2016.9. PMC 4853773. PMID 27462456.
- Zalatan, J. G.; Lee, M. E.; Almeida, R.; Gilbert, L. A.; Whitehead, E. H.; La Russa, M.; Lim, W. A. (2015). "Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds". Cell. 160 (1): 339–350. doi:10.1016/j.cell.2014.11.052. PMC 4297522. PMID 25533786.
- Morita, S.; Noguchi, H.; Horii, T.; Nakabayashi, K.; Kimura, M.; Okamura, K.; Hatada, I. (2016). "Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions". Nature Biotechnology. 34 (10): 1060–1065. doi:10.1038/nbt.3658. PMID 27571369.
- Liu, X. S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Jaenisch, R. (2016). "Editing DNA Methylation in the Mammalian Genome". Cell. 167 (1): 233–247. doi:10.1016/j.cell.2016.08.056. PMC 5062609. PMID 27662091.
- Amabile, A.; Migliara, A.; Capasso, P.; Biffi, M.; Cittaro, D.; Naldini, L.; Lombardo, A. (2016). "Inheritable Silencing of Endogenous Genes by Hit-and-Run Targeted Epigenetic Editing". Cell. 167 (1): 219–232. doi:10.1016/j.cell.2016.09.006. PMC 5039111. PMID 27662090.
- Tompkins JD. www.epigenomeengineering.com
- CRISPR Activation of Single Genes Turns Skin Cells to Stem Cells
- Liao, H. K.; Hatanaka, F.; Araoka, T.; Reddy, P.; Wu, M. Z.; Sui, Y.; Esteban, C.R.; Izpisua Belmonte, J.C. (2017). "In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation". Cell. 171 (7): 1495–1507. doi:10.1016/j.cell.2017.10.025. PMC 5732045. PMID 29224783.
- Lau, C. H.; Suh, Y. (2018). "In vivo epigenome editing and transcriptional modulation using CRISPR technology". Transgenic Research. 27 (6): 489–509. doi:10.1007/s11248-018-0096-8. PMC 6261694. PMID 30284145.
References
- Whitaker JW, Chen Z; Wang W (2014). "Predicting the human epigenome from DNA motifs". Nature Methods. 12 (3): 265–272. doi:10.1038/nmeth.3065. PMC 4344378. PMID 25240437.
- Jones, Peter A. (29 May 2012). "Functions of DNA methylation: islands, start sites, gene bodies and beyond". Nature Reviews Genetics. 13 (7): 484–492. doi:10.1038/nrg3230. PMID 22641018.
- Gaj, Thomas; Gersbach, Charles A.; Barbas, Carlos F. (2013). "ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering". Trends in Biotechnology. 31 (7): 397–405. doi:10.1016/j.tibtech.2013.04.004. PMC 3694601. PMID 23664777.
- Konermann, Silvana; Brigham, Mark D.; Trevino, Alexandro; Hsu, Patrick D.; Heidenreich, Matthias; Le Cong; Platt, Randall J.; Scott, David A.; Church, George M.; Zhang, Feng (2013). "Optical control of mammalian endogenous transcription and epigenetic states" (PDF). Nature. 500 (7463): 472–6. Bibcode:2013Natur.500..472K. doi:10.1038/nature12466. PMC 3856241. PMID 23877069.
- Maeder, Morgan L; Angstman, James F; Richardson, Marcy E; Linder, Samantha J; Cascio, Vincent M; Tsai, Shengdar Q; Ho, Quan H; Sander, Jeffry D; Reyon, Deepak; Bernstein, Bradley E; Costello, Joseph F; Wilkinson, Miles F; Joung, J Keith (2013). "Targeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins". Nature Biotechnology. 31 (12): 1137–1142. doi:10.1038/nbt.2726. PMC 3858462. PMID 24108092.
- Mendenhall, Eric M; Williamson, Kaylyn E; Reyon, Deepak; Zou, James Y; Ram, Oren; Joung, J Keith; Bernstein, Bradley E (2013). "Locus-specific editing of histone modifications at endogenous enhancers". Nature Biotechnology. 31 (12): 1133–1136. doi:10.1038/nbt.2701. PMC 3858395. PMID 24013198.
- Voigt, Philipp; Reinberg, Danny (2013). "Epigenome editing". Nature Biotechnology. 31 (12): 1097–1099. doi:10.1038/nbt.2756. PMID 24316647.
- Chen, H.; Kazemier, H. G.; de Groote, M. L.; Ruiters, M. H. J.; Xu, G.-L.; Rots, M. G. (2013). "Induced DNA demethylation by targeting Ten-Eleven Translocation 2 to the human ICAM-1 promoter". Nucleic Acids Research. 42 (3): 1563–1574. doi:10.1093/nar/gkt1019. PMC 3919596. PMID 24194590.
- Biolabs, New England. "CRISPR/Cas9 and Targeted Genome Editing: A New Era in Molecular Biology | NEB". www.neb.com. Retrieved 2016-06-07.
- "Addgene: CRISPR/Cas9 Guide". www.addgene.org. Retrieved 2016-06-07.
- McDonald, James I.; Celik, Hamza; Rois, Lisa E.; Fishberger, Gregory; Fowler, Tolison; Rees, Ryan; Kramer, Ashley; Martens, Andrew; Edwards, John R. (2016-05-09). "Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation". Biology Open. 5 (6): 866–74. doi:10.1242/bio.019067. ISSN 2046-6390. PMC 4920199. PMID 27170255.
- Hilton, Isaac B.; D'Ippolito, Anthony M.; Vockley, Christopher M.; Thakore, Pratiksha I.; Crawford, Gregory E.; Reddy, Timothy E.; Gersbach, Charles A. (2015-05-01). "Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers". Nature Biotechnology. 33 (5): 510–517. doi:10.1038/nbt.3199. ISSN 1087-0156. PMC 4430400. PMID 25849900.
- Rapid and reversible epigenome editing by endogenous chromatin regulators
- Liu, XS; Wu, H; Krzisch, M; Wu, X; Graef, J; Muffat, J; Hnisz, D; Li, CH; Yuan, B; Xu, C; Li, Y; Vershkov, D; Cacace, A; Young, RA; Jaenisch, R (2018). "Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene". Cell. 172 (5): 979–992.e6. doi:10.1016/j.cell.2018.01.012. PMC 6375087. PMID 29456084.
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. (2008). "Photoexcited CRY2 Interacts with CIB1 to Regulate Transcription and Floral Initiation in Arabidopsis". Science. 322 (5907): 1535–1539. Bibcode:2008Sci...322.1535L. doi:10.1126/science.1163927. PMID 18988809.