Elimination reaction of free radicals
An elimination reaction of free radicals is the mechanism by which free radicals can undergo an elimination reaction to form olefins.[1] Such reactions are usually not major pathways for radical mediated reactions.
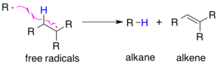

Reaction mechanisms
Radicals can undergo a disproportionation reaction through a radical elimination mechanism (See Fig. 1). Here a radical abstracts a hydrogen atom from another same radical to form two non-radical species: an alkane and an alkene.
Radicals can also undergo an elimination reaction to generate a new radical as the leaving group. For example, when polystyrene decomposes upon heating at a temperature above 300°C, a styrene monomer is generated via a radical elimination mechanism (See Fig. 2).[2] Here, the new radical is generated on the polymer chain, which can further undergo a similar type of reaction to generate more styrene molecules. This process is known as the radical mediated depolymerization of polystyrene.
Radical elimination reactions are found in enzyme-catalyzed pathways. In the dehydrogenation reaction of acyl-CoA to form enoyl-CoA, FAD accepts two protons and two electrons to form FADH2 under the catalysis of acyl-CoA dehydrogenase.[3] The mechanism involves formation of acyl-CoA β-radical that undergo elimination to form the enoyl-CoA product (See Fig. 3).
References
- Ansylen, E. V.; Dougherty, D. A. Modern Physical Organic Chemistry. p. 586, ISBN 978-1-891389-31-3
- Grassie, N.; Kerr, W. W. Trans. Faraday Soc., 1957, 53, 234-239
- Thorpe, C.; Kim, J. J.; FASEB J., 1995, 9, 718-725